How do you calculate pollen germination? A pollen grain was considered germinated when pollen tube length was at least equal to or greater than the grain diameter. Germination percentage (%) was determined by dividing the number of germinated pollen grains per field of view by the total number of pollen per field of view.
- Germination percentage was determined by dividing the number of germinated pollen grains per field of view by the total number of pollen per field of view.
- Formula for calculation of pollen grain germination.
- Pollen fertility test (%) = number of stained pollen / Total number of pollen X 100.
How do you calculate germination rate from percentage?
How is germination rate calculated? Germination Rate Calculation To calculate 5% of 10% you convert 5% to a decimal: = 0.05 You then multiply 0.05 by 10 and you get: = 0.5% Subtract and add 0.5% to 10% and you get an interval that gives you a little more room to get your hypothesis right: = 9.5% - 10.5%
How long does it take for pollen to germinate?
Pollen germination typically takes up < 8% of the duration of the progamic phase, whereas the period of pollen tube growth determines > 92% of its duration ( Williams, 2012b ).
How do you conduct a germination test?
Laboratory germination tests are normally conducted at 20°C, so if the test is to be done indoors aim to conduct it at this temperature. Count out 100 seeds (including damaged ones) and sow 10 rows of 10 seeds —the rows make it easier to count seedlings. Seeds should be sown at normal seeding depth of 2-3 cm.
How does temperature affect pollen germination in plants?
Degeneration may occur within a week at temperatures below 10 °C. In addition, cool temperatures, just before flowering at warm temperatures, can delay pollen germinability and germ-tube growth. Similar conditions can reduce fertility by disrupting aspects of ovule development ( Ebadi et al., 1995 ). Figure 3.26.

How do you calculate pollen?
Pollen sterility test (%) = number of unstained pollen / Total number of pollen X 100.
How do you do a pollen germination experiment?
This is done by dissolving 10g of sucrose as well as 10mg of boric acid in 100ml of water. Pour a few drops of this solution onto the cavity slide. Then, use a brush or fingers to gently dust a few pollen grains from the stamen of mature flowers. Let the slide set for 5 mins.
How do you measure the growth of a pollen tube?
The pollen grains i.e. the male gamete reaches the ovary through the pollen tube. The growth of the pollen tube is usually apical. The length of the pollen tube is usually measured by comparing it in terms of length at intervals of 30 minutes.
What is pollen germination?
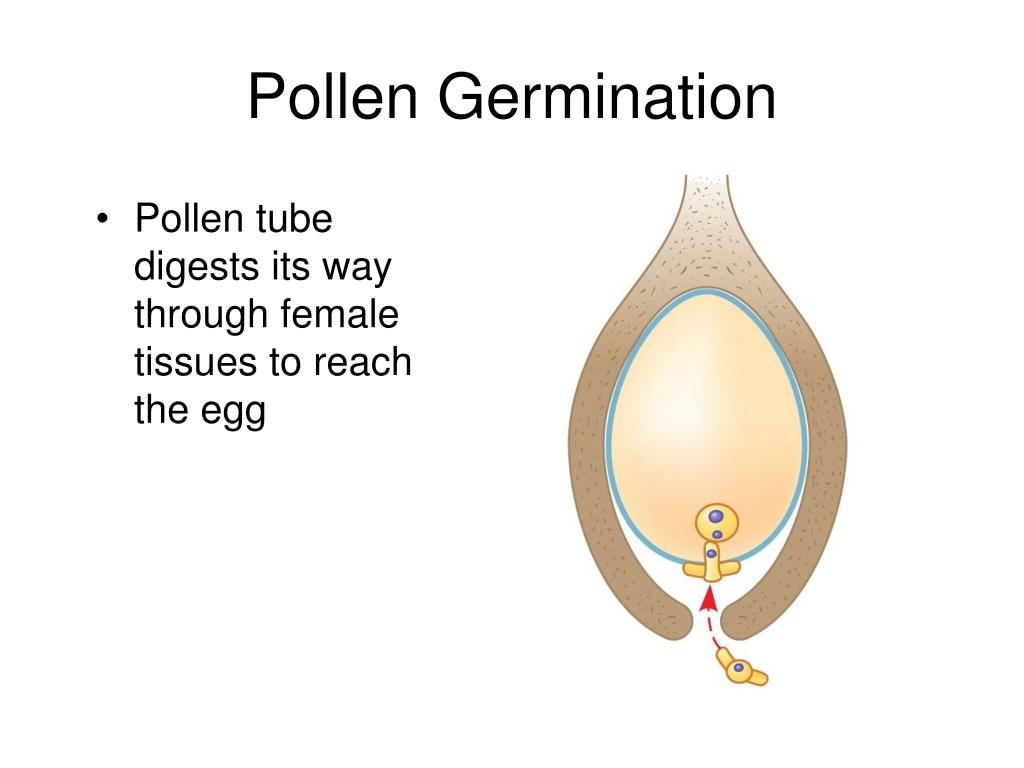
When pollen grains land on the stigma, compatible grains germinate and an elongating tube forms and grows through the aperture of the pollen grain. Pollen germination is closely related to its hydration to produce turgor, which pushes the pollen cytoplasm to protrude through the aperture to form the pollen tube.
Which solution is used for pollen germination?
SucroseSucrose is use for pollen germination as it helps in formation of pollen tubes, in vitro.
How long does it take for pollen to germinate?
The time from pollination until pollen germination ranged from <1 min to 60 h, whereas the time between pollination and fertilization in the same set of species ranged from 15 min to 13 months (Fig. 5A). There were 55 genera with 'fast' pollen germination of 30 min or less (Nepi et al.
Which of the following method is used for the measurement of pollen tube length?
Pollen tube length is most commonly determined using microscopy, which requires time-consuming scoring and measurement of individual pollen tubes.
How long does it take for pollen tubes to grow?
Typically, it took approximately 5–10 days to see the germinated pollen tubes (defined as twice the length of a pollen grain). Some pollen grains did not germinate until 10–20 days after incubation.
Which criteria is best suited to determine growth in pollen tube?
Growth of root and pollen tube is measured in terms of their length, leaf in terms of area, fruit in terms of volume and bacteria in terms of cell number.
What are the 3 stages of germination?
There are three major stages in the germination process. These are the imbibition of water, increased metabolic activity, and swelling of cells. Germination begins with the seed's imbibition (absorption) of water.
What are the two types of germination?
Based on their growing conditions and the fate of the cotyledons, the process of germination is classified into two main types: Epigeal Germination. Hypogeal Germination.
What is the difference between pollen germination and seed germination?
Answer. Pollens are very different from seeds because they are fine and powdery. They contain the micro gametophytes or the gametes (comparable to the sperm cells) of seed plants. Like ordinary seeds, pollens can also have a hard coating for the pollen grain to provide protection during movement (pollination).
How can you observe the pollen grain under microscope explain the experiment?
Take a slide and put a few drops of water on it. Take any flower like hibiscus, tridax, marigold, etc., tap it over the drop of water. We will see small dot like structures in water. These are pollen grains.
What is the conclusion for pollen germination?
During germination, the tube cell expands into a pollen tube. The pollen tube then grows towards the flower's ovule, where it releases the sperm generated in the pollen grain for fertilization. The germinated pollen grain with its two sperm cells in the adult male microgametophyte of these plants.
How do you make a pollen grain slide?
ProcedureAdd two drops of glycerol on to a clean slide.Add a small sample of the pollen (this can also be done by tapping the anther to obtain the pollen)Gently place the cover slip on the to sample at an angle to remove air bubbles.Nail polish can be used on the sides for the purposes of sealing.More items...
How does pollen germinate on stigma?
Once the pollen grains are deposited on the stigma, it starts to germinate with the absorption of nutrients and water. A small pollen tube is produced through the style to the ovary. The tube cell moves out of the pollen grain and through one of the germ pores forms a pollen tube.
Why do we need to compute for germination percentage?
A germination test determines the percentage of seeds that are alive in any seed lot. The level of germination in association with seed vigor provides a very good estimate of the potential field performance.
What is germination speed?
Germination speed is calculated as the number of days required to attain 15% germination.
What is germination capacity?
Germination capacity is the percentage of seeds that would normally germinate under optimal conditions for the species. The purpose of the germination evaluation tests is to determine the maximum germination potential of a particular batch of seeds. Seeds are planted under optimal conditions for to obtain variety.
What is pollen germination?
Pollen germination in vitro is a reliable method to test the pollen viability. It also addresses many basic questions in sexual reproduction and particularly useful in wide hybridization.
How much boric acid is needed for pollen germination?
In order to improve germination, first boric acid concentration was altered keeping Ca (NO 3) 2 at 300 ppm. At 250 ppm, maximum pollen germination was obtained (approximately 63%) and increasing or decreasing boric acid concentration over 250 ppm did not improve germination.
What is the basic medium for pollen?
A basic medium contains a sugar, calcium nitrate and boric acid to which poly ethylene glycol, vitamins, amino acids, growth regulators etc. are added to make a complete pollen germination medium. The pH and temperatures are also important factors. 2.2. Pollen germination methods.
What is the purpose of polyethylene glycol in pollen?
Polyethylene glycol (PEG): It is known to be a non-penetrating osmotic agent that decreases water potential of culture medium [ 21 ]. In pollen grains, PEG is considered to regulate the permeability of plasma membrane and to give stability to the pollen tube membrane [ 6] and to give stability to the pollen tube membrane. PEG of different molecular weight has been in pollen cultures of different species and in all cases it has a promontory effect [ 4, 6, 22 ]. Generally 10–15% of PEG was added to PGM. PEG 4000 was preferred for pigeonpea and brinjal whereas wheat and rye pollen germination satisfactorily with addition of PEG 6000.
What temperature is agarified medium in Petri dishes?
The plats were incubated in a BOD incubator with a temperature of 18°C/20.5°C depending on the pollen sample
When was pollen first observed in Portulaca?
Pollen germination in the stigmatic tissue of Portulaca was first observed as early as 1824 by Amici and later he observed the germinating pollen tube entering ovule. Pollen acts as a vehicle for the transfer of male gametes to embryo of female plant.
Can grass pollen be germinated?
Grasses pollen so far considered as recalcitrant can also be germinated in the artificial medium e.g. wheat and rye. The grass pollen is released at high moisture content (30–40%) as compared to 1–5% in the case of orthodox species. This trait makes it unsuitable for in vitro germination.
How Do You Calculate the Germination Rate?from thespruce.com
To calculate the germination rate for each type of seed, the first step is to document the number of seeds you planted and the second is to see how many of those seeds actually germinated. You can use the following formula to calculate the germination rate:
What Is Germination and Its Process?from thespruce.com
Have you ever wondered how seeds sprout? You may have seen one of those time-lapse videos online or in a movie that shows a small seedling sprouting into a bigger plant. Or, you may have planted a seed yourself in a garden and watched it grow magically in front of you.
What is the process of a plant growing from a seed?from thespruce.com
If you love planting in your garden, then you likely already know about germination, which is the process by which a plant grows from a seed. Seeds germinate and grow when the right amount of water, warmth, and soil comes together.
Can seeds germinate in the winter?from thespruce.com
If seeds are sown too late in the season, it might germinate and seem successful, but may not survive the winter as a seedling and then you are back to square one.
Popular Answers (1)
I think the partially stained pollen would be counted under sterile pollen becoz they are abnormal and even they take a little stain they could fail to germinate properly. So you can go for germination test to check their viability.
All Answers (12)
I think the partially stained pollen would be counted under sterile pollen becoz they are abnormal and even they take a little stain they could fail to germinate properly. So you can go for germination test to check their viability.
How much germination should pulse seed have?
All pulse crop seed to be used for sowing should be germination tested. Ideally only pulse seed with greater than 80% germination should be used. Germination testing can be done in a laboratory or at home.
How long does it take for a seedling to be counted?
Seedlings should be counted after 7 to 10 days when the majority of seedlings are up. Do not wait until the late ones emerge–these are the damaged, weak ones.
How is pollen germination determined?from sciencedirect.com
After initial adhesion of pollen on the stigma, pollen germination is determined by the pollen population effect: the number of pollen grains affecting pollen germination. Phytosulphokine-α (PSK-α), a sulphated pentapeptide, could stimulate pollen germination in vitro and might be the determinant of the pollen population effect ( Chen et al., 2000 ). PSK-α is a signalling molecule with multiple functions ( Matsubayashi et al., 2001 ), is released from cultured pollen with less than 2-hrs incubation and can significantly enhance germination of low-density cultured pollen ( Chen et al., 2000 ). A leucine-rich repeat receptor kinase (LRR-RK) might function as a PSK-α receptor ( Matsubayashi and Sakagami, 2000, Matsubayashi et al., 2002, Matsubayashi et al., 2006a, Matsubayashi et al., 2006b, Shinohara et al., 2007 ), but the detailed mechanism remains to be deciphered.
How do pollen grains transfer to germination?from sciencedirect.com
Pollen grains transfer from a quiescent state to a germination program when they land on the stigma. Successful landing and adhesion is the essential first step to initiate an intimate mating dialogue between both male and female gametophytes. Before tightly adhering on the stigma, pollen grains must be captured by stigma. In plants with wet stigmas, such as Lilium, the initial adhesion factors are present in the stigma-secreted exudates. Liquid surface tension on the wet stigma is suggested to be sufficient to support pollen capture ( Heslop-Harrison, 1981, Heslop-Harrison and Shivanna, 1977 ). In plants with dry stigmas, the compositions in the pollen cell walls determine the fate of the reorganization step. The pollen wall is complex, with high divergence of components among species ( Blackmore et al., 2007 ). In general, most pollen walls are composed of two major distinct layers: the exine, accumulated primarily by sporopollenin, and the intine, mainly composed of pectin and cellulose. In addition, different protein- and lipid-rich materials form a pollen coat (tryphine) anchor on the exine. Exine has a key role in the initial pollen–stigma adhesion in dry-stigma plants. Binding assay results clearly revealed that a defective pollen coat in Arabidopsis eceriferum ( cer6-2) mutated pollen does not affect initial binding significantly ( Zinkl et al., 1999 ), but removal of the pollen coat via chemical extraction showed a similar result. For example, several treatments, including salts, chaotropes, divalent cation chelators, reducing or oxidizing reagents, protease and lipase, did not disrupt the initial pollen–stigma adhesion, but certain detergents did. These results indicate that the initial pollen–stigma adhesion is likely through hydrophobic interaction caused by a primary substance in the exine and hydrophobic moiety-rich sporopollenin ( Zinkl et al., 1999 ).
What are the two ionic factors that regulate the growth of pollen?from sciencedirect.com
Two ionic factors, Ca 2+ and H + , exhibit greatly changing and oscillatory gradients in the tip of growing tubes and are considered to have pivotal roles in regulation of biological events such as actin remodelling. The tip-focused [Ca2+] gradient, observed only in growing pollen tubes, exhibits a steep decrease in level from the apex to the subapical region along the axis of pollen tubes. During tube oscillatory growth, the change in [Ca 2+] level in the extreme apex can be as much as fourfold, with a range between 700 and more than 3000 nM ( Holdaway-Clarke et al., 1997, Pierson et al., 1996 ). The [Ca 2+] gradient seems to be maintained by Ca 2+ influx at the pollen tube tip membrane through putative apical strength-activated calcium channels ( Dutta and Robinson, 2004 ). In oscillatory pollen growth, the maximal subcellular [Ca 2+] level at the tip is the same as the phase of growth rate. However, the maximal influx of [Ca 2+] at the pollen tube apex follows the growth peak and lags by about 11 seconds, which suggests that fast tube growth might change the membrane strength to increase the influx of [Ca 2+] by activating putative apical strength-activated calcium channels. However, Arabidopsis mutants defective in a cell membrane-associated Ca 2+ -ATPase are male deficient and show decreased seed setting and reduced pollen growth rates in vitro, which suggests that Ca 2+ -ATPase might be required for forming a tip-focused Ca 2+ gradient ( Schiott et al., 2004 ). The downstream signalling proteins of Ca 2+ involved in pollen tube growth, such as calmodulin-like protein (CML) and CDPKs, have been identified, which reflects the importance of Ca 2+ ( Myers et al., 2009, Yoon et al., 2006, Zhou et al., 2009 ). In Arabidopsis, a functional survey of pollen-specific calcium regulatory proteins reveals that one CML, three CDPKs and three calcineurin B-like (CBL) proteins participate in pollen tube growth. The tobacco pollen tubes overexpressing cytoplasm-localized Arabidopsis CDPK32 (AtCDPK32) show reduced length and significantly swollen tips of growing tubes. However, perturbation of a Petunia plasma membrane-localized CDPK also causes depolarization of pollen tubes ( Yoon et al., 2006 ). These studies imply tip-focused Ca 2+ regulation of polar tube growth mediated by cytoplasmic CDPK. Recently, two plasma membrane-localized Arabidopsis CDPKs, AtCDPK34 and AtCDPK17, were reported to be critical regulators of tube growth. AtCDPK34 and AtCDPK17 double mutants show reduced growth rate and failed fertilization. Interestingly, the double mutant harboring a calcium-insensitive CDPK34 transgene cannot recover fertility, which supports a mechanistic model that AtCDPK17 and AtCDPK34 induce Ca2+ signals to increase the rate of pollen tube tip growth ( Myers et al., 2009 ). Otherwise, except for the calcium-related signalling molecules mentioned above, several ABPs can be directly regulated by calcium to modulate actin-binding activity (see previous discussion). Calcium-sensitive profilin and villins/gelsolins regulating actin dynamics might be critical for oscillatory tube elongation by rhythmic [Ca2+] in the tip of pollen tubes.
What is the role of profilin in pollen growth?from sciencedirect.com
Profilin, a monomeric ABP, possesses modulating actin nucleation activity and enhances actin polymerization activity. Excess profilins leading to disturbed actin organization and inhibited pollen tube growth suggests that the proper amount of profiling is critical for pollen tube growth ( Vidali et al., 2001 ). Some profiling is sensitive to Ca 2+, which displays a concentration gradient distributed in the tip region in an oscillatory fluctuation manner; thus, the spatiotemporal regulation of profilin activity seems to persist in tube growth ( Kovar et al., 2000 ).
What are the functions of ABPs in pollen tube growth?from sciencedirect.com
To date, numerous articles have comprehensively described that several ABPs control plant development and pollen tube growth by their specific functions in actin structure regulation, such as capping, severing, bundling, branching, nucleation, polymerization and depolymerization ( Higaki et al., 2007, Hussey et al., 2006, Ren and Xiang, 2007, Staiger, 2000 ). The control of actin dynamics by ABPs in pollen tubes is usually coordinated with other regulatory factors, such as H +, Ca 2+ and PtdIns [4,5] P2, reported as having critical roles for pollen tube growth, to rapidly and precisely respond to extrinsic signals ( Foissner et al., 2002, Lovy-Wheeler et al., 2006, Monteiro et al., 2005 ). Here, we briefly introduce how the interaction of ABPs and signalling factors triggers actin remodelling to promote pollen tube growth.
What regulates pollen tube growth?from sciencedirect.com
Fig. 2. Endomembrane trafficking, small GTPases and actin remodelling regulate pollen tube growth. Three biological events, endomembrane trafficking, regulation of Rac-Rho GTPases and actin remodelling, involved in tube growth are shown. Dynamic vesicle transport through endocytosis and exocytosis in the dome of pollen tubes targets the cell membrane and cell wall materials to the apex and recycles excess membrane, extracellular matrix and the membrane-bound signalling molecule, diacyl glycerol (DAG), to the intracellular compartment (A). The dynamic distribution of phosphatidylinositol (4,5)-bisphosphate (PtdIns [4,5]P2) and its derivative lipid DAG are also shown. PtdIns-phospholipase C (PI-PLC) localized at the subapical region and shank of tubes catalyses apex-localized PtdIns [4,5] P2 into DAG and inositol 1,4,5-trisphosphate (Ins [1,4,5]P3). The membrane-bound DAG is generated at the subapical region and then retargeted to the apex by an endomembrane trafficking pathway. Two Rab small GTPases, small vesicle-localized Rab11 and Golgi-localized Rab2, participating in endomembrane transport are also shown. The activity regulation of small Rac-Rho small GTPases, which is mediated by GTPase-activating factor (GAP), guanine nucleotide-exchange factor (GEF) and guanine nucleotide dissociation inhibitor (GDI), shows specific distribution. The apical membrane-localized GEF promotes exchange of GDP to GTP to activate Rac-Rho GTPase. Activated GTP-bound Rac-Rho GTPase associating with the membrane flows to the subapical membrane by retrograde transport and meets GAP. Subapical region-resident GAP enhances intrinsic GTPase activity of Rac-Rho GTPase to change to an inactive GDP-bound form by GTP hydrolysis. Following inactivation of Rac-Rho GTPase in the subapical membrane, the cytoplasmic GDI promotes GDP-bound Rac-Rho GTPase dissociation from the GAP and recycles it to the apical membrane. GDP-bound Rac-Rho GTPase is changed to the GTP-bound form by the apical membrane-localized GEF. As mentioned here, GTP-bound Rac-Rho GTPase associates with PtdlnsPK to enhance its activity. Several actin-binding proteins (ABPs) and ionic factors whose activity is to control actin dynamics are shown in (C). The thick and stable actin cables are only in the shank of the pollen tube. In the subapical and apical regions, abundant, fine actin filaments exist and show high dynamic organization. Three ABPs, ADF, villin and gelsolin, which have actin filament severing activity, show different activity regulated by spatial distribution of calcium and proton in the tip of pollen tubes. The proton influx in the apical domain and efflux in the subapical region temporarily creates a slight alkaline banding in the apical region of pollen tubes. The ADF activity to sever actin filaments is increased by association with AIP in a slightly alkaline condition as shown in the visible form. By contrast, low-activity ADF, which is phosphorylated by the Rac-Rho GTPase pathway, associates with apical membrane-localized PtdIns [4,5]P2 and localizes in other regions, whose slightly acidic condition in pollen tubes is shown in the transparent form. The tip-focused calcium gradient is made by calcium influx in the apical domain. The activity of villin and gelsolin, which promotes actin severing, is upregulated by a high calcium condition in the tip apex, as shown in the visible form. The activities of the actin-severing factor, ADF, villin and gelsolin, are considered to provide more ends of actin filaments by actin polymerization for fast tube growth. Actin-polymerizing protein, formin, is associated with the membrane domain to polymerize thin actin filaments.
What are the proteins that regulate pollen hydration?from sciencedirect.com
In addition to lipids, certain proteins in the stigma and pollen coat have key roles in pollen hydration. Cycloheximide-treated stigma elevated the rate of incompatible pollen hydration in Brassica, which suggests that continued protein synthesis is required to regulate pollen hydration ( Sarker et al., 1988 ). Proteomic analysis of the Arabidopsis pollen coat showed that most of the corresponding genes locate in two genomic clusters: one encodes six lipases and the other contains six lipid-binding oleosin genes. All six glycine-rich proteins (GRPs) contain a consensus oleosin domain and a glycine-rich unique repetitive motif and are indispensable in pollen hydration; one example is GRP17 ( Mayfield and Preuss, 2000, Mayfield et al., 2001 ). Variation in individual repeat sequences of GRP between species and even ecotypes may evolve to form species barriers. Rapid evolution of the GRP cluster also suggests that members of this protein family are potential candidates for species-specific recognition in the pollen–stigma interaction ( Fiebig et al., 2004, Mayfield et al., 2001, Schein et al., 2004 ). Similar to GRP17, the pollen extracellular matrix (ECM) lipase 4 (EXL4) also participates in pollen hydration on the Arabidopsis stigma: exl4-1 showed a reduced pollen hydration rate, but pollen morphology and fertility were normal. EXL4 functioning in combination with GRP17 to promote the initiation of hydration implies that the oleosin domain of GRP17 may solubilize lipids, making them accessible by EXL4 and other pollen coat lipases. Therefore, changes in lipid composition at the pollen–stigma interface, possibly mediated by EXLs, are essential for efficient hydration ( Updegraff et al., 2009 ).