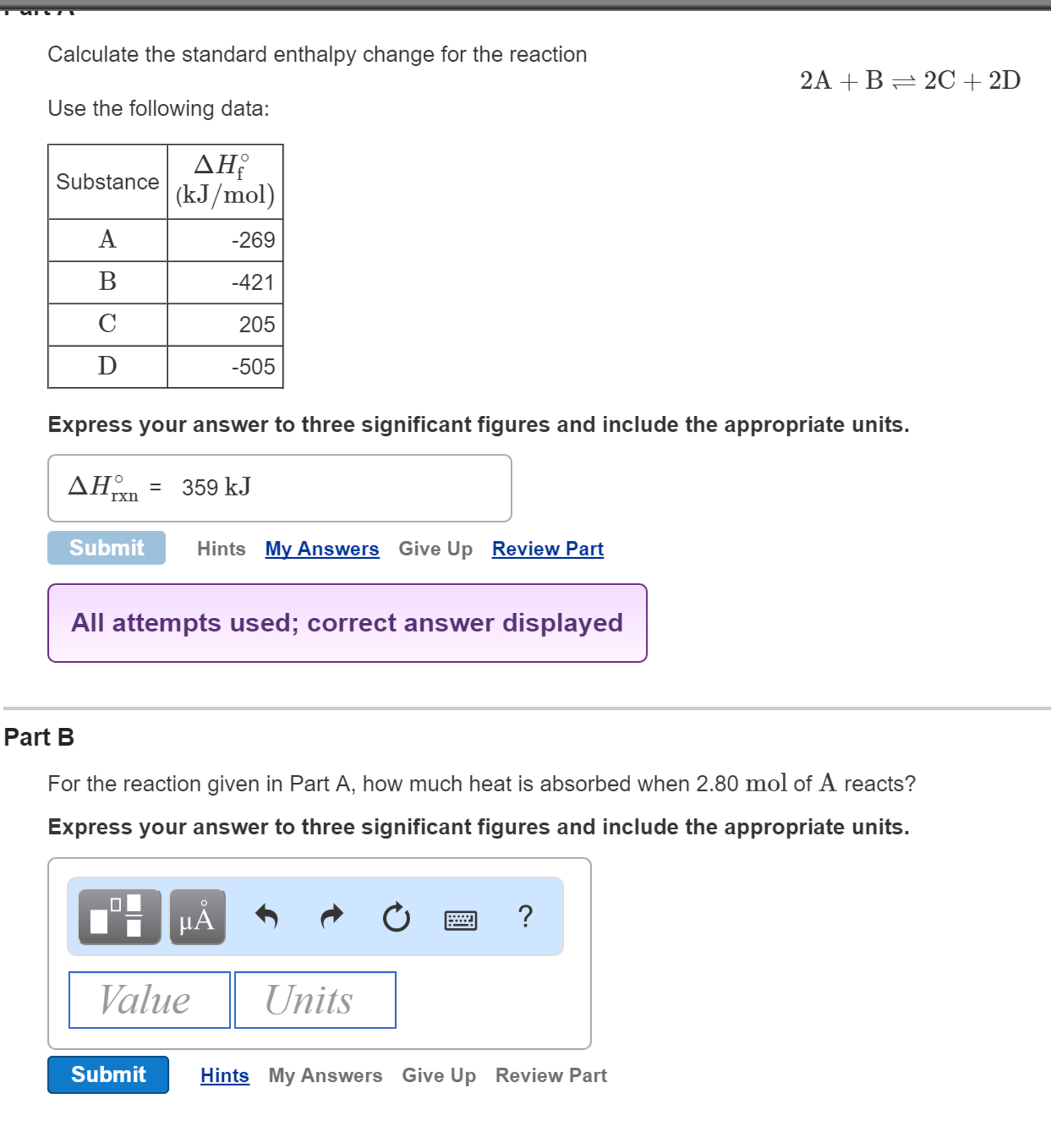
If you want to calculate the enthalpy change from the enthalpy formula:
- Begin with determining your substance’s change in volume.
- Find the change in the internal energy of the substance.
- Measure the pressure of the surroundings.
- Input all of these values to the equation ΔH = ΔQ + p * ΔV to obtain the change in enthalpy:
- Begin with determining your substance's change in volume. ...
- Find the change in the internal energy of the substance. ...
- Measure the pressure of the surroundings. ...
- Input all of these values to the equation ΔH = ΔQ + p * ΔV to obtain the change in enthalpy:
What is the formula used to calculate enthalpy?
Mar 28, 2018 · You can calculate changes in enthalpy using the simple formula: ∆H = H products − H reactants Definition of Enthalpy The precise definition of enthalpy (H) is the sum of the internal energy (U) plus the product of pressure (P) and volume (V).
How do I figure out the change in enthalpy?
May 18, 2021 · How do you calculate change in enthalpy? Once you have m, the mass of your reactants, s, the specific heat of your product, and T, the temperature change from your reaction, you are prepared to find the enthalpy of reaction. Simply plug your values into the formula H = m x s x T and multiply to solve.
How to calculate enthalpy change using a calorimeter?
Jul 20, 2021 · Calculate the enthalpy change .... Determine the amount of heat that is transferred out of the water in the calorimeter. GIVEN m = 50.0 g + 5.0 g (water + salt) = 55.0 g c = 4.18 J/gC.. The resultant solution records a temperature of 40.0°C. The heat gained by the resultant solution can be calculated using. qsolution = m c ∆T where m is the ....
How can the enthalpy change be determined?
But when it comes to calculating the change in enthalpy, you must consider both the initial and final state. Let’s assume that the pressure remains constant when the reaction occurs. In such a situation, the change in enthalpy is: ΔH = (Q₂ – Q₁) + p * (V₂ – …
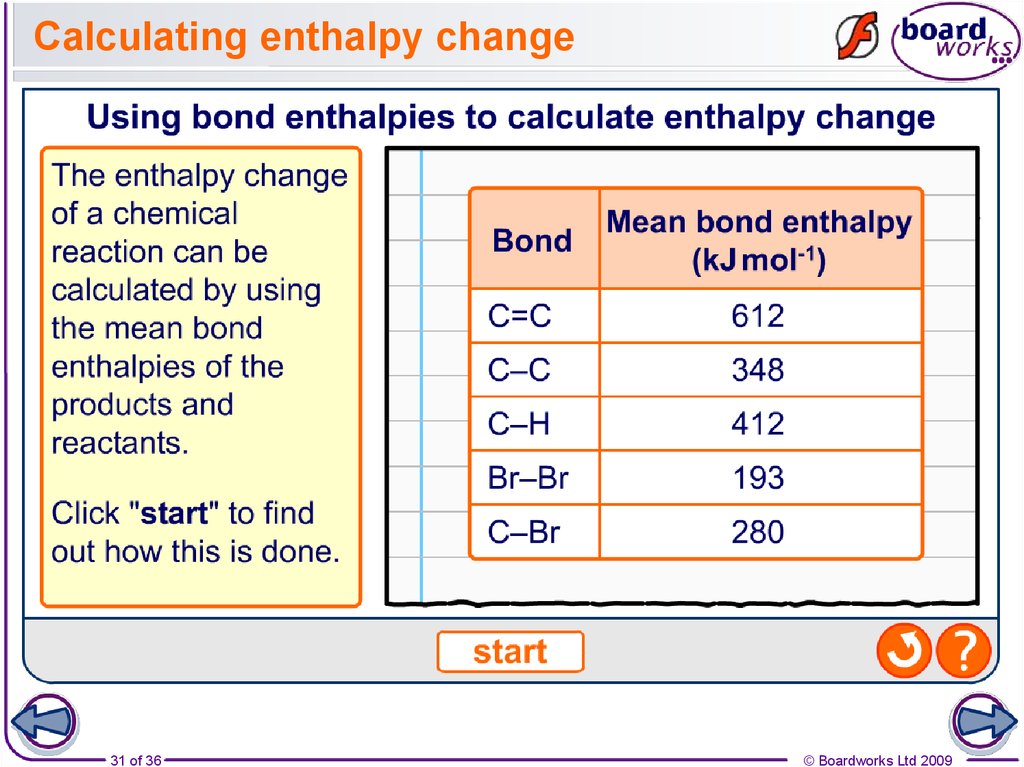
How to find the enthalpy of a chemical reaction?
To calculate the enthalpy of a chemical reaction, start by determining what the products and reactants of the reaction are. Then, find the total mass of the reactants by adding all of their individual masses together. Next, look up the specific heat value of the product.
How to test enthalpy?
1. Grab a clean container and fill it with water. It's easy to see the principles of enthalpy in action with a simple experiment. To make sure that the reaction in your experiment will take place without any foreign contamination, clean and sterilize the container that you plan to use.
What is the heat exchange between a chemical reaction and its environment?
The heat exchange between a chemical reaction and its environment is known as the enthalpy of reaction , or H. However, H can't be measured directly — instead, scientists use the change in the temperature of a reaction over time to find the change in enthalpy over time (denoted as ∆H ). With ∆H, a scientist can determine whether a reaction gives ...
How many times has wikihow been viewed?
To create this article, 24 people, some anonymous, worked to edit and improve it over time. This article has been viewed 1,201,787 times.
What are the two categories of chemicals in a chemical reaction?
Any chemical reaction involves two categories of chemicals — products and reactants. Products are the chemicals created by the reaction, while reactants are the chemicals that interact, combine, or break down to make the product. In other words, the reactants of a reaction are like the ingredients in a recipe, while the products are like ...
What happens if the sign is negative?
On the other hand, if the sign is negative, the reaction is exothermic. The larger the number itself is, the more exo- or endo- thermic the reaction is. Beware strongly exothermic reactions — these can sometimes signify a large release of energy, which, if rapid enough, can cause an explosion.
Can energy be destroyed in a chemical reaction?
Since, in a chemical reaction, energy can be neither destroyed nor created, if we know the energy required to form or break the bonds being made (or broken) in the reaction, we can estimate the enthalpy change for the entire reaction with high accuracy by adding up these bond energies.
How do you calculate change in enthalpy?
Once you have m, the mass of your reactants, s, the specific heat of your product, and T, the temperature change from your reaction, you are prepared to find the enthalpy of reaction. Simply plug your values into the formula H = m x s x T and multiply to solve. Your answer will be in the unit of energy Joules (J).
How do you find q at constant pressure?
At constant pressure, the change in the enthalpy of a system is equal to the heat flow: H=qp.
How do you find the entropy change of an ideal gas?
5:14Suggested clip 118 secondsHow To Calculate Entropy Changes: Ideal Gases – YouTubeYouTubeStart of suggested clipEnd of suggested clip
What is entropy and how do you calculate it?
Entropy is a measure of probability and the molecular disorder of a macroscopic system. If each configuration is equally probable, then the entropy is the natural logarithm of the number of configurations, multiplied by Boltzmann’s constant: S = kB ln W.
What is the formula for change in entropy?
Entropy changes (ΔS) are estimated through relation ΔG=ΔH−TΔS for finite variations at constant T.
What is the symbol for change in entropy?
Entropy is a function of the state of the system, so the change in entropy of a system is determined by its initial and final states….EntropyCommon symbolsSSI unitjoules per kelvin (J⋅K−1)In SI base unitskg⋅m2⋅s−2⋅K−1
What is entropy and its unit?
Entropy is a measure of randomness or disorder of the system. The greater the randomness, the higher the entropy. It is state function and extensive property. Its unit is JK−1mol−1.
How to calculate enthalpy?
How to use the enthalpy calculator? 1 First, enter the value of the Change in Internal Energy then choose the unit of measurement from the drop-down menu. 2 Then enter the value of the Change in Volume and choose the unit of measurement from the drop-down menu. 3 Finally, enter the value of the Pressure then choose the unit of measurement from the drop-down menu. 4 After entering all of the required values, the enthalpy calculator automatically generates the value of enthalpy for you.
When does enthalpy change?
It is the change of enthalpy which occurs when 1 mole of a substance forms from constituent elements while all the other substances remain in standard states. You can compute the enthalpy change of a given reaction under any kind of conditions from this equation.
What is the standard enthalpy of a reaction?
What is meant by the standard enthalpy of a reaction? The standard enthalpy of a reaction which has a symbol of ΔHr⦵ refers to the enthalpy change that happens in a given system when matter gets transformed by a chemical reaction. Here, all of the products and reactants stay in the standard states.
What is the difference between enthalpy and enthalpy?
This refers to the measurement of the total energy of a given thermodynamic system. It comes in the form of either volume or heat multiplied by pressure. Enthalpy is a state function which means that it only depends on a system’s equilibrium state. The change of enthalpy is the more intriguing quantity as this refers to ...
What is the enthalpy change of reactants?
In other words, the enthalpy change refers to how much heat gets evolved or absorbed when reactants transform at a given pressure and temperature into the products at exactly the same pressure and temperature.
What is the standard unit of measurement for pressure?
For pressure, use pascal as the standard unit of measurement. But you can also use kilogram per meter per second-squared or kg/ [m_s^2]. For volume, use meter cubed or m3 as the standard unit of measurement.
What does VB mean in a stoichiometric equation?
In the equation, vB refers to the stoichiometric number of B.
