How do you calculate the rate of effusion of a gas?
- rate of diffusion=amount of gas passing through an areaunit of time.
- rate of effusion of gas Arate of effusion of gas B=√mB√mA=√MB√MA.
How do you find the rate of effusion between gases?
This means that if two gases A and B are at the same temperature and pressure, the ratio of their effusion rates is inversely proportional to the ratio of the square roots of the masses of their particles: rate of effusion of A rate of effusion of B = √MB √MA rate of effusion of A rate of effusion of B = M B M A Figure 3.
What is the rate of effusion?
Recall the definition of rate of effusion: rate of effusion = amount of gas transferred time rate of effusion = amount of gas transferred time and combine it with Graham’s law:
What is Graham's Law of diffusion and effusion?
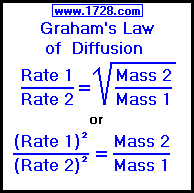
Graham's law of diffusion and effusion states the rate of diffusion or effusion for a gas is inversely proportional to the square root of the molar mass of the gas. r ∝ 1/(M)½. or. r(M)½ = constant. where. r = rate of diffusion or effusion. M = molar mass.
What is the rate at which nitrogen gas effuses?
At a particular pressure and temperature, nitrogen gas effuses at the rate of 79 mL/s. Using the same apparatus at the same temperature and pressure, at what rate will sulfur dioxide effuse?
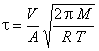
What is the effusion rate of gas?
The rate of effusion of a gaseous substance is inversely proportional to the square root of its molar mass. Thus the rate at which a molecule, or a mole of molecules, diffuses or effuses is directly related to the speed at which it moves.
How do you calculate the rate of effusion of h2?
From Graham's law, we can use the molar mass of each gas: rate of effusion of hydrogenrate of effusion of oxygen=√32g mol−1 √2g mol−1 =√16√1=41 rate of effusion of hydrogen rate of effusion of oxygen = 32 g mol − 1 2 g mol − 1 = 16 1 = 4 1 Hydrogen effuses four times as rapidly as oxygen.
What is effusion of gas?
Effusion is the movement of a gas through a tiny hole into a vacuum. We want to know the rate of effusion, which is how much gas moves through the hole per unit time. We assume that the gas particles don't bump into each other while they move through the hole (this means it's a narrow hole in a thin wall).
How do you find the rate of effusion ratio?
The ratio of the rates of effusion of two gases is equal to the square root of the inverse ratio of their molar masses or densities. The effusion rate of a gas is inversely proportional to the square root of its molar mass.
What is the effusion rate of n2?
14.65 mL/minUnder identical conditions, the rate of effusion of pure nitrogen (N2) gas is 14.65 mL/min.
What is the effusion rate of CO2?
The relative rates of difusion of CO2 and Cl2 gases is 1: 1.267 .
What is effusion with example?
Effusion is defined as a loss of material across a boundary. A common example of effusion is the loss of gas inside of a balloon over time. The rate at which gases will effuse from a balloon is affected by a number of factors.
What is effusion explain with example?
In medical terminology, an effusion refers to accumulation of fluid in an anatomic space, usually without loculation. Specific examples include subdural, mastoid, pericardial and pleural effusions.
What is diffusion and effusion of gas?
Diffusion occurs when gas molecules disperse throughout a container. Effusion occurs when a gas passes through an opening that is smaller than the mean free path of the particles, that is, the average distance traveled between collisions. Effectively, this means that only one particle passes through at a time.
What is the ratio of effusion rates of o2 and h2?
4:1Hence, the ratio of the rate of effusion of the hydrogen to that of the oxygen, will be 4:1.
What is the formula of rate of diffusion of gas?
Graham's Law Formula Graham's law states that the rate of diffusion or effusion of a gas is inversely proportional to the square root of its molar mass. See this law in equation form below. r ∝ 1/(M)½ or. r(M)½ = constant.
What is the ratio of effusion for F₂ gas to cl₂ gas?
What is the ratio of effusion for F₂ gas to Cl₂ gas? a) because rate of effusion of F2 to rate of effusion of Cl2 is 1.37:1.
What is the ratio of effusion rates of o2 and h2?
4:1Hence, the ratio of the rate of effusion of the hydrogen to that of the oxygen, will be 4:1.
How fast is the rate of effusion of h2 compared to that of o2?
4 timesThe correct answer is that Oxygen gas will effuse 4 times slower than hydrogen gas.
How do you calculate diffusion rate?
Key Equationsrate of diffusion=amount of gas passing through an areaunit of time.rate of effusion of gas Arate of effusion of gas B=√mB√mA=√MB√MA.
How much faster on average does h2 travel than o2?
4 timesThe average velocity of hydrogen molecules is 4 times greater than that of oxygen molecules.
What is the rate of nitrogen gas effusive?
At a particular pressure and temperature, nitrogen gas effuses at the rate of 79 mL/s. Using the same apparatus at the same temperature and pressure, at what rate will sulfur dioxide effuse?
How fast does hydrogen gas effuse?
Hydrogen gas effuses through a porous container 8.97-times faster than an unknown gas. Estimate the molar mass of the unknown gas.
What is the difference between diffusion and effusion?
Figure 2. Diffusion occurs when gas molecules disperse throughout a container. Effusion occurs when a gas passes through an opening that is smaller than the mean free path of the particles, that is , the average distance traveled between collisions. Effectively, this means that only one particle passes through at a time.
What is the process of moving gaseous species similar to diffusion?
A process involving movement of gaseous species similar to diffusion is effusion, the escape of gas molecules through a tiny hole such as a pinhole in a balloon into a vacuum (Figure 2). Although diffusion and effusion rates both depend on the molar mass of the gas involved, their rates are not equal; however, the ratios of their rates are the same.
Why is gas diffusion being replaced?
Because gaseous diffusion plants require very large amounts of energy (to compress the gas to the high pressures required and drive it through the diffuser cascade, to remove the heat produced during compression, and so on), it is now being replaced by gas centrifuge technology, which requires far less energy. A current hot political issue is how to deny this technology to Iran, to prevent it from producing enough enriched uranium for them to use to make nuclear weapons.
How is uranium enriched?
Gaseous diffusion has been used to produce enriched uranium for use in nuclear power plants and weapons. Naturally occurring uranium contains only 0.72% of 235 U, the kind of uranium that is “fissile,” that is, capable of sustaining a nuclear fission chain reaction. Nuclear reactors require fuel that is 2–5% 235 U, and nuclear bombs need even higher concentrations. One way to enrich uranium to the desired levels is to take advantage of Graham’s law. In a gaseous diffusion enrichment plant, uranium hexafluoride (UF 6, the only uranium compound that is volatile enough to work) is slowly pumped through large cylindrical vessels called diffusers, which contain porous barriers with microscopic openings. The process is one of diffusion because the other side of the barrier is not evacuated. The 235UF6 235 UF 6 molecules have a higher average speed and diffuse through the barrier a little faster than the heavier 238UF6 238 UF 6 molecules. The gas that has passed through the barrier is slightly enriched in 235UF6 235 UF 6 and the residual gas is slightly depleted. The small difference in molecular weights between 235UF6 235 UF 6 and 238UF6 238 UF 6 only about 0.4% enrichment, is achieved in one diffuser (Figure 4). But by connecting many diffusers in a sequence of stages (called a cascade), the desired level of enrichment can be attained.
How does diffusion work?
In general, we know that when a sample of gas is introduced to one part of a closed container, its molecules very quickly disperse throughout the container; this process by which molecules disperse in space in response to differences in concentration is called diffusion (shown in Figure 1). The gaseous atoms or molecules are, of course, unaware of any concentration gradient, they simply move randomly—regions of higher concentration have more particles than regions of lower concentrations, and so a net movement of species from high to low concentration areas takes place. In a closed environment, diffusion will ultimately result in equal concentrations of gas throughout, as depicted in Figure 1. The gaseous atoms and molecules continue to move, but since their concentrations are the same in both bulbs, the rates of transfer between the bulbs are equal (no net transfer of molecules occurs).
What is the definition of effusion rate?
Recall the definition of rate of effusion: rate of effusion = amount of gas transferred time rate of effusion = amount of gas transferred time and combine it with Graham’s law:
Which law states that the rate of diffusion and effusion of gases are inversely proportional to the square roots of?
Graham’s law of effusion: rates of diffusion and effusion of gases are inversely proportional to the square roots of their molecular masses
What happens when a mixture of gases is placed in a container with porous walls?
If a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. The lighter gases pass through the small openings more rapidly (at a higher rate) than the heavier ones (Figure 9.6.3). In 1832, Thomas Graham studied the rates of effusion of different gases and formulated Graham’s law of effusion: The rate of effusion of a gas is inversely proportional to the square root of the mass of its particles:
How do gases move?
Gaseous atoms and molecules move freely and randomly through space. Diffusion is the process whereby gaseous atoms and molecules are transferred from regions of relatively high concentration to regions of relatively low concentration. Effusion is a similar process in which gaseous species pass from a container to a vacuum through very small orifices. The rates of effusion of gases are inversely proportional to the square roots of their densities or to the square roots of their atoms/molecules’ masses (Graham’s law).
What is the difference between diffusion and effusion?
Figure 9.6.2. Diffusion occurs when gas molecules disperse throughout a container. Effusion occurs when a gas passes through an opening that is smaller than the mean free path of the particles, that is , the average distance traveled between collisions. Effectively, this means that only one particle passes through at a time.
What is the process of moving gaseous species similar to diffusion?
A process involving movement of gaseous species similar to diffusion is effusion, the escape of gas molecules through a tiny hole such as a pinhole in a balloon into a vacuum (Figure 9.6.2). Although diffusion and effusion rates both depend on the molar mass of the gas involved, their rates are not equal; however, the ratios of their rates are the same.
What happens when two cotton plugs are moistened with ammonia and the other with hydrochloric acid?
When two cotton plugs, one moistened with ammonia and the other with hydrochloric acid, are simultaneously inserted into opposite ends of a glass tube that is 87.0 cm long, a white ring of NH 4 Cl forms where gaseous NH 3 and gaseous HCl first come into contact . (Hint: Calculate the rates of diffusion for both NH 3 and HCl, and find out how much faster NH 3 diffuses than HCl.) NH3(g)+HCl(g) → NH4Cl(s) NH 3 ( g) + HCl ( g) → NH 4 Cl ( s) At approximately what distance from the ammonia moistened plug does this occur?
What happens to the effusion rate of two gases at the same temperature?
This means that if two gases A and B are at the same temperature and pressure, the ratio of their effusion rates is inversely proportional to the ratio of the square roots of the masses of their particles:
What determines the diffusion rate of a gas?
The diffusion rate depends on several factors: the concentration gradient (the increase or decrease in concentration from one point to another); the amount of surface area available for diffusion; and the distance the gas particles must travel.
What is the difference between diffusion and effusion?
Figure 2. Diffusion occurs when gas molecules disperse throughout a container. Effusion occurs when a gas passes through an opening that is smaller than the mean free path of the particles, that is , the average distance traveled between collisions. Effectively, this means that only one particle passes through at a time.
What is the process of moving gaseous species similar to diffusion?
A process involving movement of gaseous species similar to diffusion is effusion, the escape of gas molecules through a tiny hole such as a pinhole in a balloon into a vacuum ( Figure 2 ). Although diffusion and effusion rates both depend on the molar mass of the gas involved, their rates are not equal; however, the ratios of their rates are the same.
Why is gas diffusion now being replaced by gas centrifuge technology?
Because gaseous diffusion plants require very large amounts of energy (to compress the gas to the high pressures required and drive it through the diffuser cascade, to remove the heat produced during compression, and so on), it is now being replaced by gas centrifuge technology, which requires far less energy.
How does diffusion work?
In general, we know that when a sample of gas is introduced to one part of a closed container, its molecules very quickly disperse throughout the container; this process by which molecules disperse in space in response to differences in concentration is called diffusion (shown in Figure 1 ). The gaseous atoms or molecules are, of course, unaware of any concentration gradient, they simply move randomly—regions of higher concentration have more particles than regions of lower concentrations, and so a net movement of species from high to low concentration areas takes place. In a closed environment, diffusion will ultimately result in equal concentrations of gas throughout, as depicted in Figure 1. The gaseous atoms and molecules continue to move, but since their concentrations are the same in both bulbs, the rates of transfer between the bulbs are equal (no net transfer of molecules occurs).
How fast do gaseous molecules travel?
Although gaseous molecules travel at tremendous speeds (hundreds of meters per second), they collide with other gaseous molecules and travel in many different directions before reaching the desired target. At room temperature, a gaseous molecule will experience billions of collisions per second. The mean free path is the average distance ...
What is the inverse of the rate of effusion of a gas?
In 1829, Scottish chemist Thomas Graham determined through experimentation that a gas's rate of effusion is inversely proportional to the square root of the gas particle's density. In 1848, he showed that the rate of effusion of a gas is also inversely proportional to the square root of its molar mass. Graham's law also shows that the kinetic ...
What is the relationship between the rate of effusion and the molar mass of a gas?
Graham's law expresses the relationship between the rate of effusion or diffusion of a gas and that gas's molar mass. Diffusion describes the spreading of a gas throughout a volume or second gas and effusion describes the movement of a gas through a tiny hole into an open chamber.
Which law states that the rate of diffusion or effusion of a gas is inversely proportional to the square?
Graham's law states that the rate of diffusion or effusion of a gas is inversely proportional to the square root of its molar mass. See this law in equation form below.
Which isotope diffuses at a faster rate than U-238?
Through each effusion, the material passing through the pores becomes more concentrated in U-235 (the isotope used to generate nuclear energy) because this isotope diffuses at a faster rate than the heavier U-238. Cite this Article. Format. mla apa chicago.