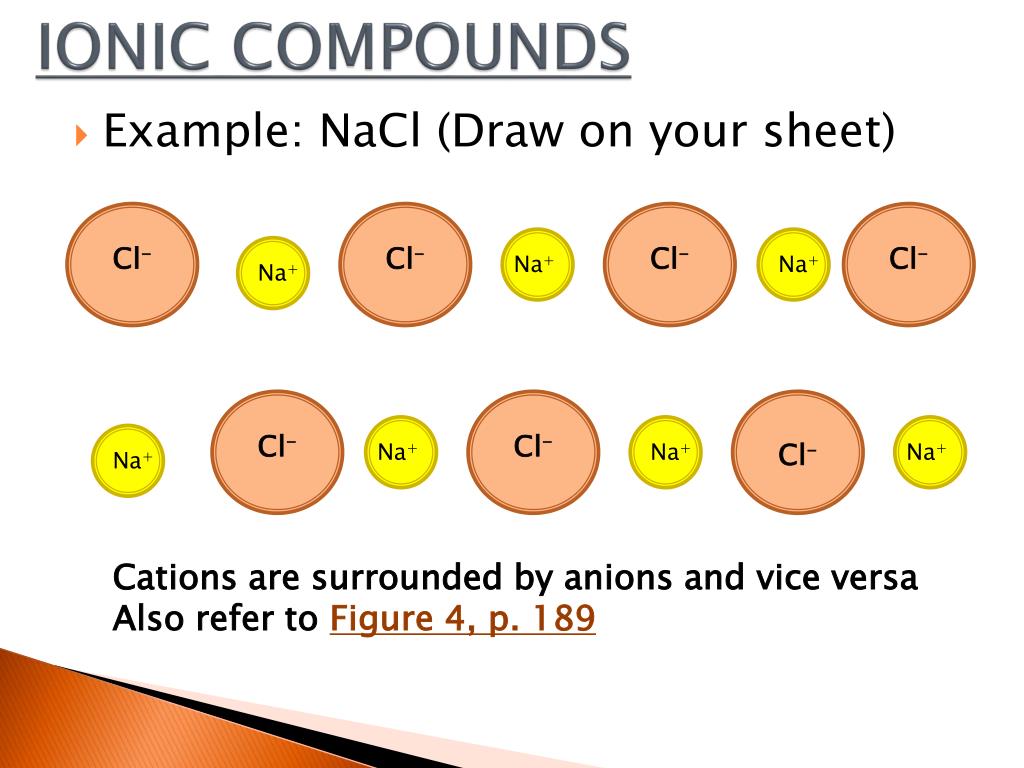
One of the ways is by donating or accepting electrons to complete their octet configuration. The bond formed by this kind of combination is known as an ionic bond or electrovalent bond. This kind of bond is formed when one atom gains electrons while the other atom loses electrons from its outermost level or orbit.
What pair or atoms can form an ionic bond?
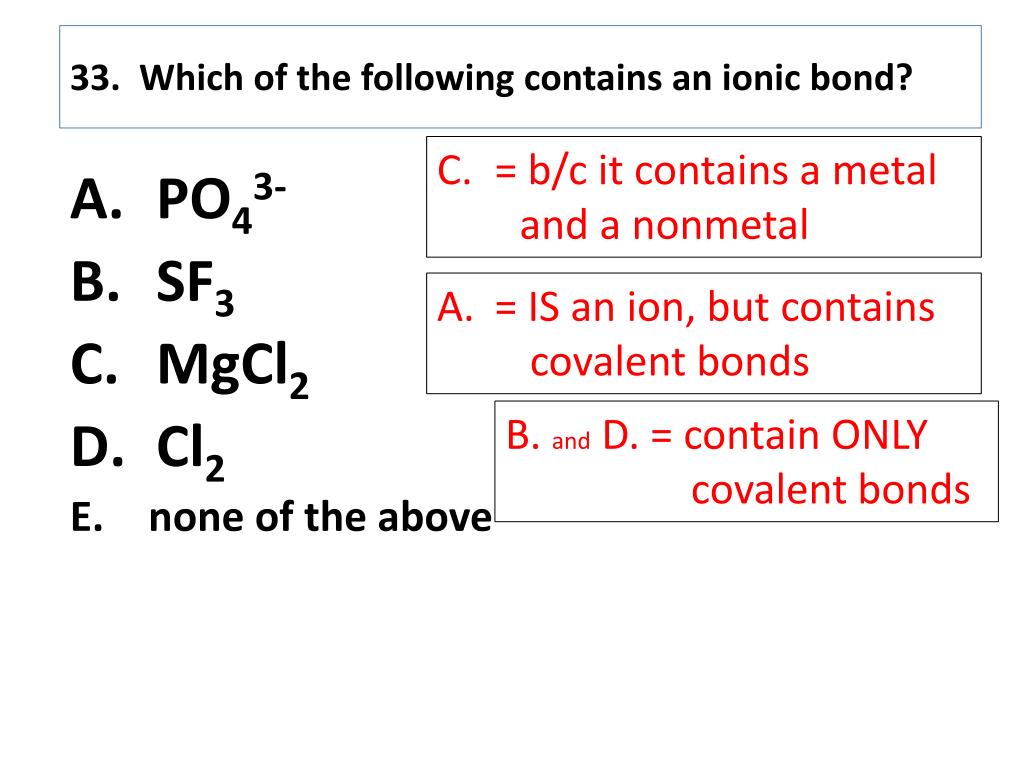
You can recognize ionic compounds because they consist of a metal bonded to a nonmetal. Ionic bonds form between two atoms that have different electronegativity values. Because the ability to attract electrons is so different between the atoms, it's like one atom donates its electron to the other atom in the chemical bond.
How do you write a formula for an ionic bond?
Writing Formulas for Ionic Compounds Containing Polyatomic Ions
- Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. Ca 2 + NO 3 −
- Transpose only the number of the positive charge to become the subscript of the anion and the number only of the negative charge to become the subscript of the ...
- Reduce to the lowest ratio. Ca 1 ( NO 3) 2
- Write the final formula. ...
Which will Forn an ionic bond?
Ionic bonds occur between metals, losing electrons, and nonmetals, gaining electrons. Ions with opposite charges will attract one another creating an ionic bond. Such bonds are stronger than hydrogen bonds, but similar in strength to covalent bonds.
What are ionic bonds and how are they formed?
ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom.
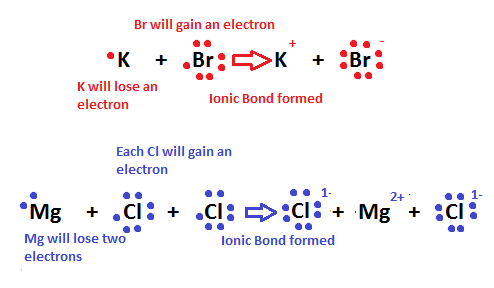
How are ionic bonds formed step by step?
The atom losing one or more electrons becomes a cation—a positively charged ion. The atom gaining one or more electron becomes an anion—a negatively charged ion. When the transfer of electrons occurs, an electrostatic attraction between the two ions of opposite charge takes place and an ionic bond is formed.
How do you write an ionic bond formula?
5:4011:43Writing Ionic Formulas: Introduction - YouTubeYouTubeStart of suggested clipEnd of suggested clipIf I don't write anything after it means 1 so 3 potassium 1 nitrogen the correct formula for this isMoreIf I don't write anything after it means 1 so 3 potassium 1 nitrogen the correct formula for this is K 3 n.
How do you make ionic bonds easy?
0:002:23Simple Ionic Bonding - YouTubeYouTubeStart of suggested clipEnd of suggested clipThis tutorial we'll look at ionic compounds formed when two elements form an ionic bond.MoreThis tutorial we'll look at ionic compounds formed when two elements form an ionic bond.
How do you write an ion?
When writing the symbol for an ion, the one- or two-letter element symbol is written first, followed by a superscript. The superscript has the number of charges on the ion followed by a + (for positive ions or cations) or - (for negative ions or anions). Neutral atoms have a charge of zero, so no superscript is given.
How do you write formulas?
5:1510:22Writing Chemical Formulas For Ionic Compounds - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd in the 1 not including the negative sign we're going to write it as a subscript to the left. SoMoreAnd in the 1 not including the negative sign we're going to write it as a subscript to the left. So this is going to be a l1 cl3 now there's no point in writing a subscript of a one.
How is an ionic bond formed example?
When a positively charged ion forms a bond with a negatively charged ion, one atom donates electrons to the other, this is known as an ionic bond. The chemical molecule Sodium Chloride is an example of an ionic bond.
How are ionic bonds formed quizlet?
ionic bonds form when electrons are transferred from one atom to another atom. ions of different elements can combine by forming ionic bonds . positive ions & negative ions form when atom s lose or gain electrons. Covalent bonds form when atoms share electrons.
What is an ionic bond 6th grade?
0:042:54What are Ionic Bonds? | Properties of Matter | Chemistry | FuseSchoolYouTubeStart of suggested clipEnd of suggested clipNow we will learn about ionic bonds ions as you already know are formed from the loss or gain ofMoreNow we will learn about ionic bonds ions as you already know are formed from the loss or gain of electrons. Electrons however do not suddenly appear or disappear.
What is an ionic bond? Explain with an example?
When a positively charged ion forms a bond with a negatively charged ion, one atom donates electrons to the other, this is known as an ionic bond....
What kind of force is present in ionic bonds?
Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from another. For both atoms involved, this...
How do you find ionic formulas?
To obtain an ionic compound’s formula, first determine the cation and record its symbol and charge. Then, write down the anion’s symbol and charge...
Are ionic bonds between two nonmetals?
Consider whether each element is a metal or a nonmetal to anticipate the sort of bond that will form between them. In general, nonmetals form coval...
How do you break ionic bonds?
Ionic chemicals dissolve in polar solvents like water because they are polar. When polar solvents disrupt the ionic bonds, they dissolve. By dissol...
What is a covalent bond?
A covalent bond is formed by the sharing of electrons between participating atoms. In it, each atom contributes an equal number of electrons for bo...
Can an ionic bond form between two metals?
No, the ionic bond can not form between two metals. An ionic bond is formed between positive and negative ions or a metal and a non-metal by the do...
How are ionic bonds formed between atoms?
Ionic bonds are formed by the complete transfer of electrons between two atoms. An atom that gains an electron acquires a negative charge and is kn...
Do ionic bonds have a high melting point?
Yes, ionic bonds have a high melting point. Ionic bonds are formed by the complete transfer of electrons held together by a strong electrostatic fo...
What is an ionic bond explained with an example?
When the electronegativity difference between the two atoms is large, they can attain octets by the complete transfer of one or more electrons from...
What are 3 examples of an ionic bond?
The three examples of ionic bonds are Lithium Fluoride, Sodium Chloride, and Lithium Bromide.
Are ionic bonds strong?
Ionic bonds are stronger than covalent bonds due to the coulombic attraction between ions of opposite charges.
How do you explain ionic bonding?
Ionic bonding is the complete transfer of valence electron(s) between atoms. It is a type of chemical bond that generates two oppositely charged ions.
What are the 4 properties of ionic compounds?
They form crystals. They have high melting points and high boiling points. They have higher enthalpies of fusion and vaporization than molecular co...
What is an ionic bond?
An ionic bond is a force of attraction between a positive and a negative charged ion. These oppositely charged ions are usually produced when a metal transfers its electron to a nonmetal. For example, when an electron is removed from a neutral sodium atom, the sodium atom becomes a positively charged sodium ion (Na + ), ...
Why are ionic bonds similar to covalent bonds?
Although some atoms share electrons to form covalent bonds and others transfer electrons to form ionic bonds, these bonds are still similar in strength because they both involve a force of attraction between unlike charges. Compounds that are formed as a result of ionic bonding are usually called ionic compounds.
What force holds sodium and chloride together?
Since opposite charges attract, the sodium and chloride ions will be held together by a strong attractive force called an electrostatic force. Chemists, however, call this electrostatic force an ionic bond. Generally, ionic bonds form between metals and nonmetals. The metals usually have low ionization energy, while the nonmetals have high electron ...
What is Ionic Bond?
When the electronegativity difference between the two atoms is large, they can attain octets by the complete transfer of one or more electrons from one atom to the other and can be linked together by an attractive force known as an ionic bond or electrovalent bond.
Born-Haber cycle
Lattice energy is the energy released when one mole of an ionic crystal is formed by the close packing of gaseous cations and anions.
Covalent Character in Ionic Bond
Several ionic compounds possess partial covalent character and resemble more to covalent compounds in their properties. For example, LiCl is an ionic compound but it is more soluble in organic solvents than in water.
Ionic Bond FAQs
Ans.3 Ionic bonds are stronger than covalent bonds due to the coulombic attraction between ions of opposite charges.
How are the constituent ions of an ionic compound held together?
In any crystal, the constituent ions of the ionic compound are held together by electrostatic forces of attraction. The stronger the forces of attraction, the higher is the lattice energy and the more stable is the compound. This electrostatic force of attraction is determined by Coulomb’s Law.
What is the ionic bond of table salt?
We are all quite familiar with table salt that we use to add flavour to food. We also know that chemically, table salt is sodium chloride in which sodium and chloride ions are bonded together by ionic bonds. Various factors affect the formation of these ions and consequently ionic bonding, which ultimately gives rise to ionic compounds. The factors are –
What is the term for the transfer of electrons from an electronegative element to an atom of an electronegative element
The chemical bond that is formed between 2 atoms through the transfer of one or more electrons from the electropositive or metallic element to the atom of an electronegative or non-metallic element is called an ionic or electrovalent bond .
What is the bond between calcium and oxygen?
Similarly, metal-like calcium loses two of its outermost electrons to a non-metal-like solid-state, oxygen resulting in an ionic bond between calcium and oxygen forming Calcium oxide (CaO). 1. A metal always forms the cation, whereas a non-metal always forms the anion. 2.
Why are ionic compounds brittle?
Ionic compounds are brittle – When an external force is applied to the crystals of an ionic compound, it shatters into pieces. This happens because, in the crystals of sodium chloride, the Na + ions and Cl – ions are lined up against each other in a lattice with a strong electrostatic force of attraction.
What is the conductivity of ionic compounds?
Electrical conductivity – In solid-state, ionic compounds are generally non-conductors of electricity. When heated to a temperature above their melting point, the electrostatic force of attraction between the ions breaks, and the ions become free to move. These free ions can now allow the passage of electricity. 5.
What is the ionization energy of sodium?
Consider the formation of sodium ion (Na +) from sodium atom. The ionization energy of sodium is about 500kJ / mol, which is quite low. It can easily lose electrons and get converted into a sodium ion. This ion can further take part in ionic bond formation with other anions such as Cl –, Br –.
How are ionic bonds formed?
Ionic Bonding. Ionic bonds are formed by the combination of positive and negative ions; the combination of these ions form in numerical combinations that generate a neutral (zero charge) molecule.
Where are the bonding electrons located?
The bonding electrons are called the VALENCE electrons and they are the electrons that are found in the outermost shell of the atom. In the periodic table below, you can see diagrams of each element that shows how many valence electrons it possesses. Conveniently, the Group Number at the top of each column in the periodic table also gives ...
What type of bonding is a covalent bond?
Ionic and Covalent Bonding. There are primarily two forms of bonding that an atom can participate in: Covalent and Ionic. Covalent bonding involves the sharing of electrons between two or more atoms. Ionic bonds form when two or more ions come together and are held together by charge differences. So how do you know what kind ...
How many valence electrons does chlorine have?
If that same hydrogen bonded to Chlorine, the hydrogen would get the two electrons it needs to be complete and the chlorine which has 7 valence electrons would get the one more to fulfil its octet. See above. Now that you can form covalent compounds we need to go over how to name these compounds.
How many electrons does hydrogen have to have to have a full valence shell?
There are several exceptions to the octet rule however: Hydrogen (H) only requires 2 electrons to have a full valence shell since it only needs to be like Helium (He).
Why do you not use the Greek prefix in ionic nomenclature?
This is because as chemists we know the number since the charge the ions take on is predictable. So to sum up the process for identifying, writing and naming compounds:
Why is it easier to lose electrons than to gain electrons?
This is because it is easier energetically for those elements to lose 1, 2, or 3 electrons than it would be for them to gain 5, 6 or 7 electrons. The gain or loss of an electron generally requires energy and once you exceed the gain or loss of 3 electrons the energy cost is simply too high for most atoms to accomplish.
How it Works
Using the Ionic Bonding Concept Builder is quite simple. There are three different activities. Each activity involves a different set of tasks ... and a different set of directions.
Earning Stars
When you start up any of the difficulty levels, the number of questions for that activity are shown in the Progress Report area of the Concept Builder. You will notice that there is either a Yellow or a Red background for each question number. When starting up a difficulty level, the background color is Yellow.
Getting Help
You will notice that there is a Help Me button underneath the Progress Report. You should learn to use this feature of the program. It's how you can turn an exercise in answering questions into an exercise in learning. Tapping on the Help Me button will open a page with help that is specific to each question.
What is the bond between two oppositely charged ions?
The ionic bond involves a transfer of electrons. The metal atom loses one or more electrons to form a positive ion (cation). The nonmetal atom gains one or more electrons to form a negative ion (anion). The attraction between these two oppositely charged ions is known as ionic bond.
How to find the number of electrons in an atom?
For neutral atoms, the number of electrons will be equal to the atomic number for that element. Place those electrons in shells until you have used up all the electrons. Begin with the innermost shell - it only gets 2 electrons as discussed in the previous section.
How many electrons does sodium have?
A sodium atom has a total of 11 electrons - 2 in the innermost shell, 8 in the second shell, and one lone electron in its valence shell . To fill its valence shell, it would need to acquire 7 electrons - a difficult task for a metal like sodium that is a known electron-loser.
How many electrons does the innermost shell hold?
Each successively larger shell can hold more electrons. So the innermost shell has a single s orbital and can hold at most 2 electrons.
Where are electrons located?
All About Electrons, Shells, and Valence Electrons. We understand electrons to be located in regions of space surrounding the nucleus that we refer to as electron shells. There are a series of concentric electron shells surrounding an atom's nucleus, each of varying size. The shells themselves contain orbitals.
Where do the next 8 electrons go in the atom?
The next 8 electrons (electron #11-18) go in the third shell; again there are 8 places to put these. If there are still more electrons to place in shells, then start placing them in the fourth electron shell. This method works really well for the first 20 element.
Does oxygen gain electrons?
And so oxygen will be happy if it can gains 2 more electrons to have a full octet of outer shell electrons. If it does, then it becomes a 2- ion: the O 2- ion. So non-metals such as fluorine and oxygen gain electrons in order to achieve a full octet of valence shell electrons.