
How to convert 12 g of oxygen gas into a mole - Quora. You can “convert” 12g of O2 gas into 1 mol O2 gas by adding 32g-12g = 20g O2 to it. Answer” Mol O2 = 12g/32g/mol = 0.375 mol O2 gas.
How do you convert from moles to grams?
There are three steps to converting moles of a substance to grams:
- Determine how many moles are given in the problem.
- Calculate the molar mass of the substance.
- Multiply step one by step two.
How many moles are there in O2?
Therefore 1 mole of oxygen gas is actually 1 mole of O2 molecules. Each molecule of O2 is made up of 2 oxygen atoms. So 1 mole of O2 molecules is made up of 2 moles of oxygen atoms. Therefore 1 mole of oxygen gas contains 2 moles of oxygen atoms. And 0.4 moles of oxygen gas contains 0.8 moles of oxygen atoms.
How do you convert from molecules to grams?
- Find the molar mass of subtances (AgNO3)
- Find AgNO3 molecules
- Formula to convert from molecules to grams is (AgNO3 number of molecules) / (6.022 x 10^23) x (substance's molar mass in grams/mol) *AgNO3 be the number of molecules or moles ...
How many grams are in one mole?
One mole contains 1827.5087 grams. Thus, one mole of barium contains 5.5367E-4 grams or 0.000553770419408789 ounces. The molecular weight of barium is 137.9. To convert from grams to milligrams, multiply the number of grams by 1370.0. To convert from milligrams to grams, divide the number of milligrams by 3.350.

How many grams of O2 are in a mole?
The mass of oxygen equal to one mole of oxygen is 15.998 grams and the mass of one mole of hydrogen is 1.008 g.
How do you calculate moles of O2?
0:232:28How to Convert Moles of O2 to Grams - YouTubeYouTubeStart of suggested clipEnd of suggested clipTable o2 oxygen is 16.00 grams per mole and we have two oxygens here two oxygen atoms. So we'llMoreTable o2 oxygen is 16.00 grams per mole and we have two oxygens here two oxygen atoms. So we'll multiply that and we get 32.00 grams per mole those are the units.
How do you convert grams to mole?
To correctly estimate the number of moles, n , of a substance of a specific mass, m , (in grams), you need to follow the grams to moles formula: n = m / M , where, M is the molar mass of this material.
What is the moles of 02?
The molar mass of O2 is 32 gram per mole.
How many moles are in 50g of O2?
1 Answer. There are 3 moles of O in 50 g.
How do you convert 12 grams of oxygen to moles?
Number of moles in 12g O2 = Given mass/ Molar mass = 12/32 = 0.375 mole.
How many grams are in a mole?
The molar mass of atoms of an element is given by the standard relative atomic mass of the element multiplied by the molar mass constant, 1 × 10−3 kg/mol = 1 g/mol.
How many grams are in 1 mole?
The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. 12.00 g C-12 = 1 mol C-12 atoms = 6.022 × 1023 atoms • The number of particles in 1 mole is called Avogadro's Number (6.0221421 x 1023).
How do you convert grams to moles in stoichiometry?
1:373:47Grams to Moles Stoichiometry - YouTubeYouTubeStart of suggested clipEnd of suggested clipBut in this case we're only going to go grams to moles to moles. So we're only going to use theMoreBut in this case we're only going to go grams to moles to moles. So we're only going to use the first two conversion factors. And this is going to end up giving us moles of what we want.
Is O2 1 mol or 2 mol?
O might also mean oxygen as the element without specifying its chemical form; O2 can only mean dioxygen gas. Anyway, 1 mole of oxygen gas contains 2 mole of oxygen atoms.
How many moles are in 40 grams of O2?
∴ There are 1.25 moles of O2 in 40g of oxygen gas.
How many grams are in 3 moles of O2?
1 Answer. 3 moles of Oxygen atoms weigh 48.00 grams.
How do I calculate moles?
The unit is denoted by mol.The formula for the number of moles formula is expressed as.Given.Number of moles formula is.Number of moles = Mass of substance / Mass of one mole.Number of moles = 95 / 86.94.
How do you find the molar mass of o2?
15.999 uOxygen / Atomic mass
What is the formula for moles?
Worked Example: moles = mass ÷ molar mass (n=m/M)
How many oxygen molecules are in o2?
Oxygen is found naturally as a molecule. Two oxygen atoms strongly bind together with a covalent double bond to form dioxygen or O2.
How to calculate moles of a compound?
The number of moles you have of a compound can be calculated by dividing the number of grams of the compound by the molecular mass of the compound. The formula looks like this: moles = grams of compound/molar mass of compound.
What is the molecular weight of oxygen?
For example, the molecular weight of oxygen is 15.99.
How to find moles in an element?
Solve the equation. Using a calculator, divide the number of grams by the molar mass. The result is the number of moles in your element or compound.
How to find molar mass of a mol?
Divide the milligrams by 1000 to get x/1000 g. Then, divide the grams by molar mass to get (x/1000 g) / (y g/mol). This gives you x/ (1000y) mol.
How to calculate molecular mass?
The molecular mass of a substance is calculated as the number of atoms of each element multiplied by the atomic weight of that element. Knowing the molecular mass is necessary to convert grams to moles. Multiply the number of atoms each element contributes to the compound by the atomic weight of that element.
What is a mole in chemistry?
Moles are a standard unit of measurement in chemistry that take into account the different elements in a chemical compound. Often, amounts of compounds are given in grams and need to be converted to moles. This conversion can help give you a clearer picture of the number of molecules you're working with rather than dealing with weight, ...
How to find the atomic mass of an element?
Write down the atomic weight of each element. A periodic table is the easiest way to find the atomic weight of an element. Once you locate the element on the table, the atomic weight is usually found underneath the symbol for that element. The atomic weight, or mass, or an element is given in atomic mass units (amu).
How many grams of hydrogen peroxide are in 0.700 moles?
Multiply the molar mass by the number of moles to get the grams: grams of hydrogen peroxide = (34.016 grams/mol) x (0.700 mol) = 23.811 grams. There are 23.811 grams of hydrogen peroxide in 0.700 moles of hydrogen peroxide.
How to calculate molar mass?
Calculate the molar mass by multiplying the number of atoms of each element in the compound (its subscript) times the atomic mass of the element from the periodic table.
How are compounds weighed?
Compounds are weighed using scales to yield grams. It's often necessary to convert grams to moles for chemistry calculations. Peter Muller / Getty Images
What are the two units used to express the amount of matter in a sample?
Grams and moles are two units to express the amount of matter in a sample. There is no "conversion formula" between the two units. Instead, you must use atomic mass values and the chemical formula to do the conversion.
How many moles of O2 are in a gram?
How many moles O2 in 1 grams? The answer is 0.031251171918947. We assume you are converting between moles O2 and gram. You can view more details on each measurement unit: molecular weight of O2 or grams. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles O2, or 31.9988 grams.
How to calculate molar mass?
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.
How to find molar mass?
Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
What is formula weight?
Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights.
Is molar mass the same as molecular mass?
This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes.
How to calculate moles from grams? - Grams to moles formula
To correctly estimate the number of moles, n, of a substance of a specific mass, m, (in grams), you need to follow the grams to moles formula:
How to convert grams to moles: an example
Take a look at the example of how to calculate moles from grams. Imagine you have 6 liters of pure water:
How many grams are in a molar mass of oxygen?
Find the molar mass of oxygen from the periodic table = 15.999 grams.
How to find grams in a mole?
To find how many grams are in a mole of a compound involves extra steps. First, determine the molar masses of the compound's component elements. Next, multiply the molar masses of these elements by the subscript following them in the compound' formula.
Why is the mole used in chemistry?
The mole is a convenient method to deal with substances because of the one-to-one conversion between amus and grams. Also, it is much more practical to measure a mole of atoms or molecules than to manipulate individual particles.
What is the mass of one mole of a substance in grams?
Molar mass is the mass of one mole of a substance in grams.
How many grams are in a mole of lithium?
The mass of one mole of lithium is 6.938 gram s. If a sample of lithium (Li) weighs 100.0 grams, how many moles of lithium are in the sample?
Why do scientists use moles?
Scientists use moles because moles are an easy and consistent way to work with large numbers of particles. In addition, using moles ensures that comparisons are being made between equal numbers of atoms or molecules. Using grams in calculations instead of moles would be unwieldy and confusing.
How to find the molar mass of an element?
To find the molar mass of an element, refer to the periodic table and find the element's atomic mass. This numeric value in grams is the molar mass of the element. The molar mass of oxygen is 15.999 g because the atomic mass of one atom of oxygen is 15.999 amu. Oxygen has a molar mass of 15.999 g.
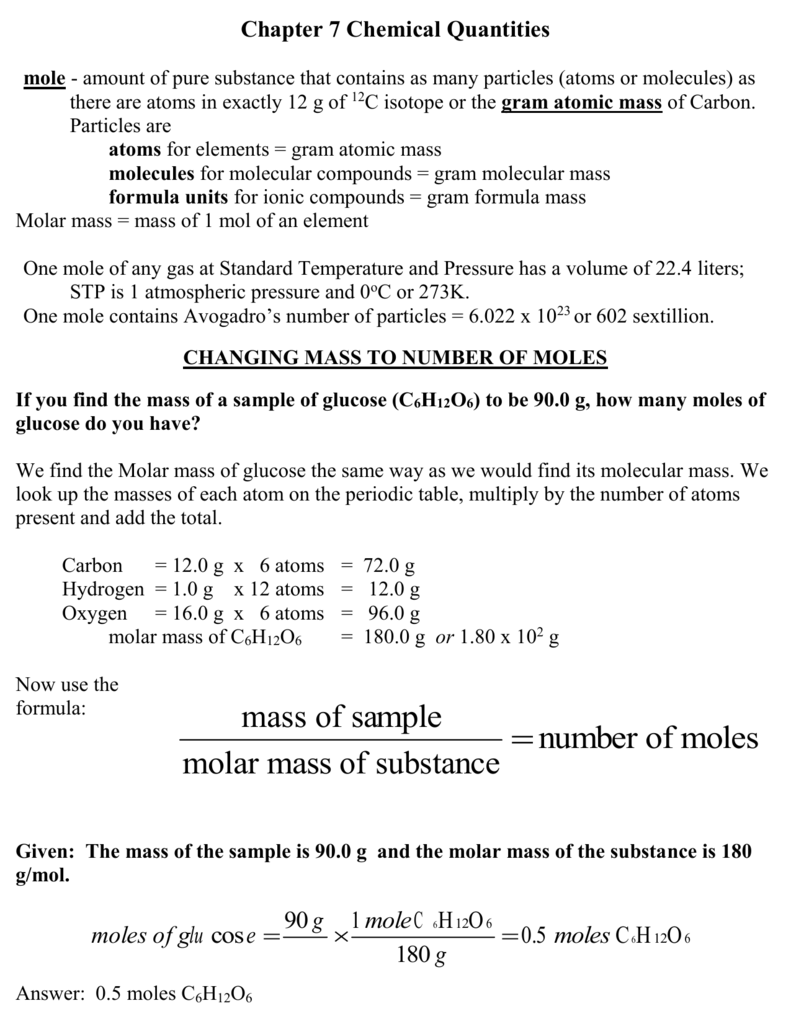
Grams to Moles Conversion Problem
- Gather the necessary tools for solving a chemistry problem. Having everything you need easily accessible will simplify the process of solving the assigned problem. You will need the following: A pencil and paper. Calculations are easier to solve when you write them out. Be sure to show all your steps to get full credit. A periodic table. You will need to be able to find …
- Identify the elements in the compound that you need to convert into moles. The first step in c…
Moles to Grams Conversion Problem
Performing Grams and Moles Conversions
Sources
- Here is another example showing how to convert moles to grams. Problem Determine the mass in grams of 3.60 mol of H2SO4. Solution First, look up the atomic masses for hydrogen, sulfur, and oxygen from the periodic table. The atomic mass is 1.008 for H, 32.06 for S, and 16.00 for O. The formula mass of H2SO4is: 2(1.008) + 32.06 + 4(16.00) = 98.08 Thus, one mole of H2SO4we…