A hydrate is any compound that has absorbed water molecules from its environment and included them in its structure. There are three types of hydrates: inorganic, organic, and gas (or clathrate) hydrates.
How do you name a hydrate formula?
An example of a hydrate formula is C a C l 2 ⋅ 2 H 2 O. The dot separating the C a C l 2 from the two water molecules isn't a multiplication symbol. It shows that the water molecules aren't bonded to the compound, and it's therefore a hydrate. In order to properly name a hydrate, first you give the name of the salt.
How do you identify hydrated ionic compounds?
Hydrated ionic compounds (i.e., hydrates) have a specfic number of water molecules in their chemical formulas. In the solid, these water molecules (also called "waters of hydration") are part of the structure of the compound. Rule 1.
Why don't hydrates have water molecules in the formula?
Because the water molecules aren't part of the compound's actual structure, this affects the way chemical formulas of inorganic hydrates are written. An example of a hydrate formula is C a C l 2 ⋅ 2 H 2 O. The dot separating the C a C l 2 from the two water molecules isn't a multiplication symbol.
What is an example of a hydrate?
An example of a hydrate formula is C a C l 2 ⋅ 2 H 2 O. The dot separating the C a C l 2 from the two water molecules isn't a multiplication symbol. It shows that the water molecules aren't bonded to the compound, and it's therefore a hydrate.
What are hydrates in chemistry?
How to write out the formulas of inorganic hydrates?
What Is the Hydrate Chemistry Naming System?
What Are Common Examples of Hydrates?
How to name a hydrate?
What is the prefix for dihydrate?
What is an organic hydrate?
See 4 more topics
About this website

How do you tell if a substance is a hydrate?
For a compound to be a true hydrate, it has to show all properties of true hydrates, including evolution of water upon heating, solubility of its anhydrous residue in water and reversibility in the color of the residue back to the color of the hydrate when dissolved in water.
How do you classify a hydrate?
There are three types of hydrates: inorganic, organic, and gas. Inorganic hydrates are by far the most common type of hydrate compound. There are specific rules for writing out the formulas and names of inorganic hydrates. For formulas, the salt's formula is written first, then a dot, then the water molecules.
How does a compound become a hydrate?
Hydrates form naturally when ionic compounds are exposed to air and make bonds with water molecules. Specifically, the bond is formed between the cation of the molecule and the water molecule. The water that remains is usually known as water of hydration or water of crystallization.
How do you test a hydrate?
One of the easiest ways to test your hydration is through bathroom frequency and urine color. Your urine should be light yellow and you should be emptying your bladder on average 5-8 times per day. Another way to determine hydration levels (especially after a run) is a sweat test.
What elements make up hydrate?
In organic chemistry, a hydrate is a compound formed by the hydration, i.e. "Addition of water or of the elements of water (i.e. H and OH) to a molecular entity".
What is a hydrate and how does it form?
Hydrates are formed when water and light end natural gases come into contact at certain temperature and pressure conditions. These gas hydrates are crystals formed by water with natural gases and associated liquids, in a ratio 85 % mole water to 15 % hydrocarbons.
Why do some compounds form hydrates?
Hydrates are usually more thermodynamically stable than anhydrous salts, so they form spontaneously when anhydrous salts are exposed to water, like atmospheric humidity. Waters of hydration help hydrated salts form well-ordered crystals.
How is a hydrate different from other chemical compounds?
1) How is a hydrate different from other chemical compounds? It has water molecules loosely attached to it. These water molecules can typically be removed through heating (a process called “dehydration”. Hydrates usually involve ionic compounds with transition metals as the cation.
What type of bond is a hydrate?
Hydrates are crystalline solids comprising water molecules linked by hydrogen bonds in a tight polyhedral cage structure.
What is a hydrate in chemistry?
hydrate, any compound containing water in the form of H2O molecules, usually, but not always, with a definite content of water by weight. The best-known hydrates are crystalline solids that lose their fundamental structures upon removal of the bound water.
What is a hydrate formula?
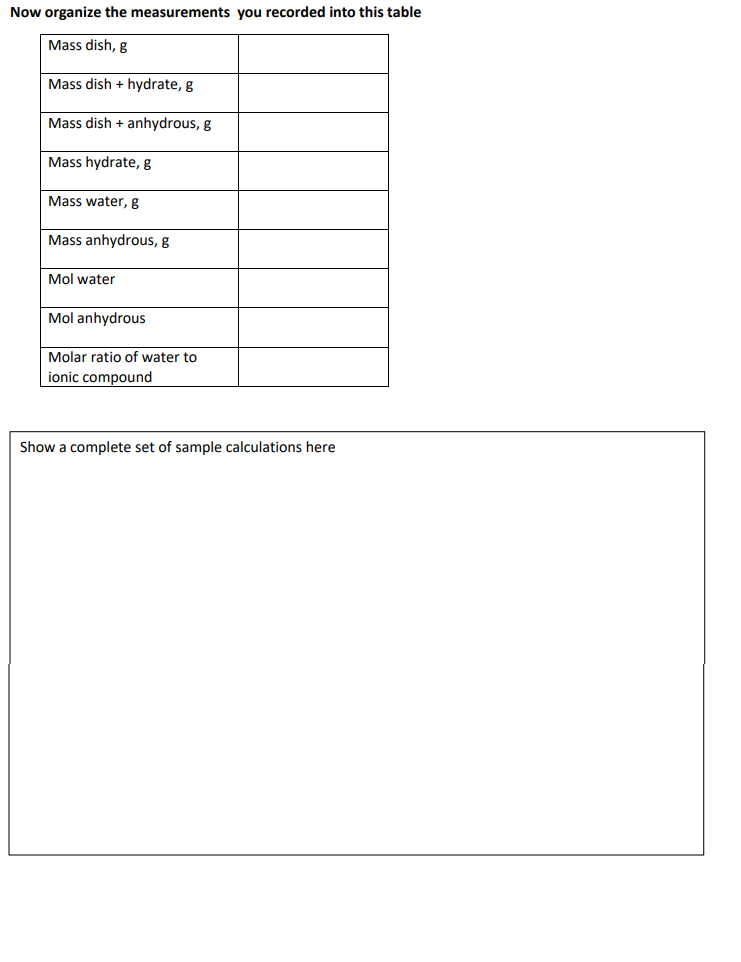
Formula of a Hydrate (Anhydrous Solid⋅xH2O) In order to determine the formula of the hydrate, [Anhydrous Solid⋅xH2O], the number of moles of water per mole of anhydrous solid (x) will be calculated by dividing the number of moles of water by the number of moles of the anhydrous solid (Equation 2.12.
How would you test an unknown crystalline compound to determine if it was a hydrate?
A true hydrate would produce water during heating. However, when the compound was dissolved in water it formed a yellow-brown solution but started out colorless. If it were a true hydrate there would be no color change when dissolved in solution because of the reversible nature of the reaction.
Hydrates in Everyday Life Flashcards | Quizlet
Study with Quizlet and memorize flashcards terms like Na2CO3, LiCl * 4H20, CoCl2 * 6H20 and more.
Naming hydrates - Chemistry Resource
A crystalline compound that contains chemically bound water molecules in definite proportions is called a hydrate. Greek prefixes are used to indicate the number of water molecules in the formula unit.. Rules for naming hydrates:
Hydrate -Definition, Types, Process, Facts and FAQs
Learn about hydrate topic of Chemistry in details explained by subject experts on vedantu.com. Register free for online tutoring session to clear your doubts.
Hydrate Definition & Meaning - Merriam-Webster
hydrate: [noun] a compound formed by the union of water with some other substance.
What are hydrates in chemistry?
What are hydrates in chemistry? The hydrate definition is that it's a compound with extra water molecules that are part of its structure . These water molecules have been absorbed from its environment. There are three types of hydrates: inorganic, organic, and gas. Inorganic hydrates are by far the most common type of hydrate compound.
How to write out the formulas of inorganic hydrates?
For formulas, the salt's formula is written first, then a dot, then the water molecules. For hydrate chemistry names, the salt's name is written out first, then a prefix depending on the number of water molecules, then the word "hydrates.".
What Is the Hydrate Chemistry Naming System?
An example of a hydrate formula is C a C l 2 ⋅ 2 H 2 O . The dot separating the C a C l 2 from the two water molecules isn't a multiplication symbol. It shows that the water molecules aren't bonded to the compound, and it's therefore a hydrate.
What Are Common Examples of Hydrates?
What are hydrates that are commonly used outside the chemistry world? Below are five common hydrate examples. All of these are inorganic hydrates.
How to name a hydrate?
In order to properly name a hydrate, first you give the name of the salt. The second part of the name begins with a prefix. The prefix is determined by the number of water molecules in the hydrate which is itself determined by the salt. You then add the word "hydrate" to the prefix to give the complete hydrate name. For the formula given above, it's hydrate name is calcium chloride dihydrate. The prefix "di" is used because it has two water molecules.
What is the prefix for dihydrate?
For the formula given above, it's hydrate name is calcium chloride dihydrate. The prefix "di" is used because it has two water molecules. Below are the prefixes for different numbers of water molecules. Number of H20 molecules. Prefix. 0.5. hemi-.
What is an organic hydrate?
Organic Hydrates: An organic hydrate is created when a water molecule is added to a carbonyl group of an aldehyde or ketone. In organic hydrates, the water molecules have chemically reacted with the compound and bonded to it.