
See more

How to find the number of neutrons in an atom?
You can find the number of neutrons if you know the isotope of the atom. Simply subtract the number of protons (the atomic number) from the mass number to find the remaining neutrons.
What are the protons, neutrons, and electrons in an atom?
Key Takeaways: Number of Protons, Neutrons, and Electrons. Atoms are made of protons, neutrons, and electrons. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. A neutral atom has the same number of protons and electrons (charges cancel each other out).
How many protons does a hydrogen atom have?
It's easy to get a hydrogen atom with one proton and one neutron (deuterium), yet you won't find a helium atom with an atomic weight of 2 because this would mean the helium atom had two protons and zero neutrons! If the atomic weight is 4.001, you can be confident the atom is helium, with 2 protons and 2 neutrons.
How are elements defined?
Each element is defined by the number of protons found in each of its atoms. No matter how many electrons or neutrons an atom has, the element is defined by its number of protons. In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen).
Why is the periodic table decimal?
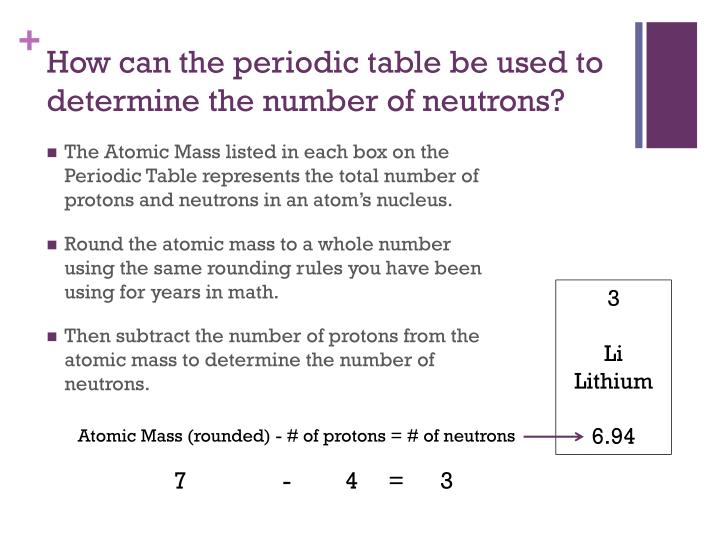
Each atom has an integer number of neutrons, but the periodic table gives a decimal value because it is a weighted average of the number of neutrons in the isotopes of each element. So, what you need to do is round the atomic weight to the nearest whole number to get a mass number for your calculations.
What does it mean when an ion has a 2+ charge?
If an ion has a 2+ charge, like Zn 2+, this means there are two more protons than electrons. If the ion has a 1- charge (simply written with a minus superscript), then there are more electrons than the number of protons. For F -, the number of protons (from the periodic table) is 9 and the number of electrons is:
What element has 2 protons?
The element of an atom with 2 protons is always helium. If you are given the atomic weight of an atom, you need to subtract the number of neutrons to get the number of protons. Sometimes you can tell the elemental identity of a sample if all you have is the atomic weight.
What is the nuclide in the middle of the atom?
The Nuclide Notation. The letter (s) in the middle is the symbol of the element. The number on the bottom left corner is the atomic number, which tells you the number of protons. The number on the upper left corner is the mass number, which is equal to the neutrons and protons added together. Lastly, the charge is on the upper right corner.
What does it mean when an atom has no charge?
Lastly, the charge is on the upper right corner. If there isn ’t any number or signs, then it means that atom has no charge and is neutral.
How to find the number of protons in an atom?
Finding the Number of Protons. The number of protons in an atom is equal to the atomic number of the element. For example, let’s use oxygen. According to the periodic table, oxygen has the atomic number eight. The atomic number is located above the element’s symbol. Since oxygen has an atomic number of eight, there must be eight protons total.
What is the atomic number of an element?
Its atomic mass is 15.999 atomic mass units (amu) and its atomic number is 8. When we subtract 8 from 15.999, we will get 8. Also, it should be noted that the number of neutrons for an element may vary. Some elements have isotopes, which have different masses and therefore different numbers of neutrons.
How many electrons are in oxygen?
This means the number of electrons and the number of protons in an element are equal. Therefore, the number of electrons in oxygen is 8. Moreover, since these two subatomic particles, electrons and protons, have opposite charges, they cancel out and keep the atom neutral.
Where is the atomic number on the periodic table?
The easiest way to find the atomic number, is to look on a periodic table, the atomic number is in the upper left corner, or is the largest number on the square.
What is the name of the negatively charged subatomic particles located in orbitals surrounding the nucleus?
Electrons : Negatively charged subatomic particles located in orbitals surrounding the nucleus. Atomic Mass: A weighted average of the number of neutrons and protons present for all isotopes. Atomic Number: Number of protons present in an atom.
How many protons are there in the atomic number?
The atomic number is 11. So that means that there are 11 protons. How ever many protons there are there will be the same amount of electrons. The mass is 22.9. Round the number to 23 and then subtract the amount of protons from 23 and then you have the amount of neutrons.
How many picometers are there in platinum?
A measured value for the atomic radius of platinum atoms was determined to be 143 picometers. Based on Table S, what is the percent error of this meas …
CORE Concepts
Covered in Other Articles
Vocabulary
- Protons: Positively charged subatomic particles located in the nucleus of an atom.
- Neutrons: Neutrally charged subatomic particles located in the nucleus of an atom.
- Electrons: Negatively charged subatomic particles located in orbitals surrounding the nucleus.
- Atomic Mass: A weighted average of the number of neutrons and protons present for all isotopes.
How to Find The Atomic Number
- The atomic number of an element is simply the number of protons in its nucleus. The easiest way to find the atomic number, is to look on a periodic table, the atomic number is in the upper left corner, or is the largest number on the square.
Finding The Number of Protons
- The number of protons in an atom is equal to the atomic number of the element. For example, let’s use oxygen. According to the periodic table, oxygen has the atomic number eight. The atomic number is located above the element’s symbol. Since oxygen has an atomic number of eight, there must be eight protons total. Moreover, the number of protons nev...
Finding The Number of Neutrons
- The number of neutrons in an atom can be calculated by subtracting the atomic number from the atomic mass. Both of these numbers can be found on the periodic table. The atomic number is listed above the symbol of the element whereas the mass number is placed below. Let’s keep using oxygen as our example. Its atomic mass is 15.999 atomic mass units (amu) and its atomi…
Finding The Number of Electrons
- The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged species. This means the number of electrons and the number of protons in an element are equal. Therefore, the number of electrons in oxygen is 8. Moreover, since these two subatomic particles, electrons and protons, have opposite charges, they cancel out and keep the atom neut…
Further Reading