
A single-displacement reaction, is a chemical reactionin which one (or more) element(s) replaces an/other element(s) in a compound. It can be represented generically as: A + B-C → A-C + B. This will mostoften occurif A is more reactive than B, thus giving a more stable product.
What are some examples of single replacement reactions?
A single replacement reaction occurs when one element replaces another in a single compound. This type of reaction has the general equation: A + BC → B + AC. An example of a single replacement reaction occurs when potassium (K) reacts with water (H 2 O). how are single replacement reactions used in our daily lives? One everyday item that we use that is the result …
What are the types of single replacement reactions?
Apr 13, 2022 · Single-displacement reactions involve the swapping or replacement of one cation with another cation or one anion with another anion. Here are two important tips for determining the products of a...
What is the formula for single replacement reaction?
Definition of single replacement (or single displacement) reactions. Predicting and determining the products using the reactivity series.
How to solve single replacement reactions?
The following episode looks at the type of reaction called Single Displacement reactions. These reactions involve single molecules in the reactants and a different single molecule in the products. Keep in mind that if a metal is single in the reactants, the other metal will become single in the products (AS LONG AS if it follows the Activity series for metals).

How do you write a single-displacement reaction?
A single-replacement reaction is a chemical reaction in which one element replaces another in a compound. F₂ + 2NaCl → 2NaF + Cl₂, where F replaces Cl in NaCl.
What is a single-displacement reaction give an example?
Single-Displacement Reaction Examples The reaction between zinc metal and hydrochloric acid to produce zinc chloride and hydrogen gas is an example of a single-displacement reaction: Zn(s) + 2 HCl(aq) → ZnCl2(aq) + H2(g)Jun 27, 2019
What are the four types of chemical reactions?
in biomedical sciences and is a science writer, educator, and consultant. She has taught science courses at the high school, college, and graduate levels. The four main types of chemical reactions are synthesis reactions, decomposition reactions, single-displacement reactions, and double-displacement reactions.
What is a single displacement reaction?
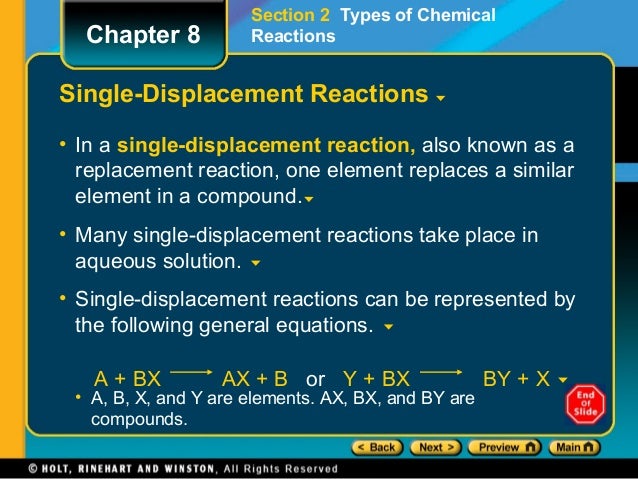
A single-displacement reaction is a chemical reaction where one reactant is exchanged for one ion of a second reactant. It is also known as a single-replacement reaction. Single displacement reactions take the form: A + BC → B + AC.
What is the reaction between zinc and hydrochloric acid?
The reaction between zinc metal and hydrochloric acid to produce zinc chloride and hydrogen gas is an example of a single-displacement reaction: Another example is the displacement of iron from an iron (II) oxide solution using coke as a carbon source:
What happens when two compounds react?
Usually, when two compounds react, both cations or both anions will change partners, producing a double-displacement reaction .
What are displacement reactions?
Applications of displacement reactions 1 It is used in thermite welding. In which aluminum displaces iron from its oxide. 2 It is sued in steel making. In which carbon displaces iron from its oxide. 3 It is largely used in extraction of metals. 4 It is used in acid indigestion. 5 It is used in flame photometry.
What is the type of reaction in which part of one reactant is displaced by another reactant?
The type of reaction in which part of one reactant is displaced by another reactant is called displacement reaction. It is also called a replacement reaction. As replacement of one ion of reactant takes place by another ion of reactant.
What are some examples of double displacement reactions?
Generally, it can be represented as follows –. Example of double displacement reaction – Reaction between silver nitrate and sodium chloride is an example of double displacement reaction. The reaction given below –.
Why are metals reactive?
The reactivity of metals is because of their incomplete outer orbitals or due to their electronic configuration. Metals form positively charged ions as they tend to lose electrons. Metals with high atomic numbers tend to be more reactive as their electrons are far from the positively charged nucleus.
What is a reactivity series?
What is Reactivity Series? Reactivity series is the series of metals based on their reactivity from highest to lowest. So, reactivity series of metals can be defined as a series of metals, in order of reactivity from highest to lowest. It is also known as activity series.
What is single displacement?
A single displacement reaction which is also called as single replacement reaction is a kind of oxidation-reduction chemical reaction when an ion or element moves out of a compound, i.e., one element is replaced by the other in a compound.
What is displacement reaction?
What is a Displacement Reaction? A displacement reaction is the one wherein the atom or a set of atoms is displaced by another atom in a molecule. For instance, when iron is added to a copper sulphate solution, it displaces the copper metal.
What happens when chlorine is added to water?
When chlorine is added in its gaseous form (or as a gas dissolved in water) to the solution of sodium bromide, the chlorine acquires the place of bromine. Since chlorine is more reactive than bromine, it displaces bromine from sodium bromide, and the solutions turn blue.
What is double displacement? What are some examples?
What is double displacement give example? Between two aqueous ionic compounds, there is a precipitation reaction to form a new insoluble ionic compound. Here is an example of a reaction to form (soluble) potassium nitrate and (insoluble) lead iodide between lead (II) nitrate and potassium iodide.
What is neutralization reaction?
What is a Neutralisation reaction? A neutralization reaction is a chemical reaction in which an acid and a base react to form a salt and water. The pH of the salt formed depends on the pH of the reacting acids and bases.
Steps to Identify A Single Displacement Reaction
- Step 1:Identify if the reaction takes place between and elemental species and a compound. Step 2:Identify if the elemental species exchanged with one of the elements of the compound.
Equations & Definitions For Identifying A Single Displacement Reaction
- Single Displacement Reaction:A reaction where an elemental species exchanges places with another element out of a compound. Elemental Species:A reactant or product that is composed of one element. This does not mean it contains only one atom. Compound:A reactant or product that is composed of more than one element. Single Displacement Generic Reaction:{eq}E + CX \right…
Example Problem 1 - Identifying A Single Displacement Reaction
- Which of the following is a single displacement reaction? A. {eq}MgCl_2+SnSO_4\rightarrow MgSO_4+SnCl_2{/eq} B. {eq}Br_2+2KI\rightarrow I_2 +2KBr{/eq} C.{eq}CH_4+2O_2\rightarrow CO_2 + 2H_2O{/eq} D. {eq}Zn^{2+}+Cu\rightarrow Zn+Cu^{2+}{/eq} Step 1:Identify if the reaction takes place between and elemental species and a compound. A single displacement reaction wi…
Example Problem 2 - Identifying A Single Displacement Reaction
- Which of the following is a single displacement reaction? A. {eq}NH_3 + H^+ \rightarrow NH_4^+{/eq} B. {eq}2H_2O\rightarrow O_2 +2H_2{/eq} C. {eq}H^++OH^-\rightarrow H_2O{/eq} D. {eq}HBr + Cl^- \rightarrow HCl + Br^-{/eq} Step 1:Identify if the reaction takes place between and elemental species and a compound. All reactions in this example take place between an elemen…
single-displacement Reaction Definition
single-displacement Reaction Examples
- The reaction between zinc metal and hydrochloric acid to produce zinc chloride and hydrogen gas is an example of a single-displacement reaction: Another example is the displacement of iron from an iron(II) oxide solution using coke as a carbon source:
Recognizing A single-displacement Reaction
- When you look at the chemical equation for a reaction, a single-displacement reaction is characterized by one cation or anion trading places with another to form a new product. It's easy to spot when one of the reactants is an element and the other is a compound. Usually, when two compounds react, both cations or both anions will change partners, producing a double-displace…