
What is cell notation?
Which half cell is described first?
How are the anode and cathode separated?
Where to write concentrations of dissolved species?
What is the difference between a positive and negative cell potential?
See 2 more
About this website
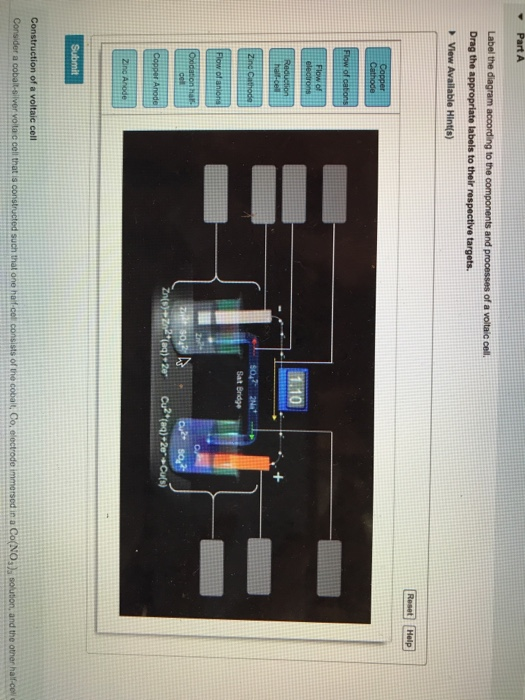
How do you make a cell diagram in chemistry?
2:284:32Chemical Thermodynamics 11.3 - Cell Diagrams - YouTubeYouTubeStart of suggested clipEnd of suggested clipThem. So any electrochemical cell we can write a cell diagram. For it's just a diagram of the twoMoreThem. So any electrochemical cell we can write a cell diagram. For it's just a diagram of the two half cells the reaction arrow separated by a single bar.
How is a cell diagram written?
The anode is placed on the left and the cathode is placed on the right. Individual solid, liquid, or aqueous phases within each half-cell are written separated by a single bar. Concentrations of dissolved species can be written in the parentheses after the phase notation (s, l, g, or aq).
How are cells represented in chemistry?
Cell notation The cell is represented by the rule that metals are written first and then the metal ions that are present in the electrolyte. And these two need to be separated by a vertical line. For example Zn | Zn2+.
How do you construct a simple cell?
A simple cell consists of two solid electrodes placed in an electrolyte connected together by an electrical conductor such as wire. The two electrodes must be two different metals. The electrolyte can be acid solution, alkaline solution, salt solution or even a fruit such as orange or lemon.
Is water included in cell diagrams?
Re: H2O in Galvanic Cell Diagram They're not included in the cell diagrams because technically with aqueous solutions, they are by definition a solute with water so it'd be redundant to add water.
Is cathode or anode written first?
By convention in standard cell notation, the anode is written on the left and the cathode is written on the right. So, in this cell: Zinc is the anode (solid zinc is oxidised). Silver is the cathode (silver ions are reduced).
What is the symbol for a cell?
The symbol for a cell has two parallel lines at right angles to the connecting wires. The thinner line marks the positive terminal of the cell and the thicker, shorter, line marks the negative terminal. A battery (more than one cell) is drawn as a series of cells, each usually adding 1.5 volts.
How do you represent a cell in chemistry class 12?
A galvanic electrochemical cell can be represented by writing the anode on the left-hand side and cathode on the right-hand side. The anode is represented by the solid phase or the metal first, followed by the electrolyte.
How do you write a cell in line notation?
1:2912:04Cell Notation Practice Problems, Voltaic Cells - ElectrochemistryYouTubeStart of suggested clipEnd of suggested clipSolution along with the concentration. Then you can have a double vertical line which separates theMoreSolution along with the concentration. Then you can have a double vertical line which separates the half cell on the left from the half cell on the right.
What is a simple cell in chemistry?
A simple cell is a source of electrical energy. The simplest design consists of two electrodes made from metals of different reactivity immersed in an electrolyte and connected to an external voltmeter by wire, creating a complete circuit. A common example is zinc and copper.
What is a simple cell?
a neuron, most commonly found in the striate cortex, that has a receptive field consisting of an elongated center region and two elongated flanking regions. The response of a simple cell to stimulation in the center of the receptive field is the opposite of its response to stimulation in the flanking zones.
How do you describe a cell?
What is a cell? A cell is a mass of cytoplasm that is bound externally by a cell membrane. Usually microscopic in size, cells are the smallest structural units of living matter and compose all living things. Most cells have one or more nuclei and other organelles that carry out a variety of tasks.
Which side of a cell is positive in a diagram?
The positive terminal of a cell is the long line and the negative terminal is the short line. If there is a two-cell battery, then the long line on the end is the positive terminal of the battery. The short line on the opposite end is the negative terminal of the battery.
How do you read half cells?
Cell Notation Rules Within a given half-cell, the reactants are specified first and the products last. The description of the oxidation reaction is first, and the reduction reaction is last; when you read it, your eyes move in the direction of electron flow. Spectator ions are not included.
Is the cathode positive or negative?
The Anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during and electrochemical reaction. The Cathode is the positive or oxidizing electrode that acquires electrons from the external circuit and is reduced during the electrochemical reaction.
What is the formula of E cell?
The overall cell potential can be calculated by using the equation E0cell=E0red−E0oxid.
23.6: Calculating Standard Cell Potentials - Chemistry LibreTexts
Calculating Standard Cell Potentials. In order to function, any electrochemical cell must consist of two half-cells.The table below can be used to determine the reactions that will occur and the standard cell potential for any combination of two half-cells, without actually constructing the cell.
What is a cell diagram?
A cell diagram is Chemistry's short-hand for representing a galvanic cell (voltaic cell) . The usual convention for writing a cell diagram is: (that is, the oxidation reaction is shown on the left) . When the diagram is read from left to right it shows the direction of the electron flow through the galvanic cell (voltaic cell).
Where is the anode on a galvanic cell diagram?
The anode is shown on the left hand side of the diagram. (that is, the oxidation reaction is shown on the left) . When the diagram is read from left to right it shows the direction of the electron flow through the galvanic cell (voltaic cell). For the galvanic cell shown to the right: Anode reaction (oxidation):
Re: Writing a cell diagram, electrodes
Platinum is an inert conductor that must be in the cell with the half reaction that has no conducting solids. For example, Zn (s) is a conducting metal so Pt is not needed in the Zn cell. In the other cell, N and O aren't conducting metals so Pt added to conduct electrons from cell to cell. Pt doesn't participate in the reaction.
Re: Writing a cell diagram, electrodes
Don't forget to write out the states when you're doing the cell diagram. That will make it much easier.
Re: Writing a cell diagram, electrodes
For the cell diagrams, why don't we include the numbers of the electrode (for ex: Midterm 2009 we put lH+l when there are 2H+'s? And is it acceptable to place a comma between two of the ions rather than another line?
Re: Writing a cell diagram, electrodes
Only place a line if the compounds/anions/cations/elements are in different phases! A comma is used in-between if the pieces are in the same phase. Between ions, there should be commas because ions are aqueous.
Re: Writing a cell diagram, electrodes
Thank you so much! Would there be a time when you don't need to place the Pt in the equation? If there is, how do you know?
Re: Writing a cell diagram, electrodes
You don't need Pt (s) if the electrode is a conducting, solid metal such as Zn (s) and Cu (s). So look for the clues--first you need something in the solid phase. Then, check if it is a metal.
What happens when an electric current flows into the cathode of an electrolytic cell?
When an external electric current flows into the cathode of the electrolytic cell, the resulting negative charge attracts the dissociated positive ions present in the electrolyte. This results in the deposition of the positively charged ions onto the cathode.
What are the components of an electrolytic cell?
The three main components of electrolytic cells include the cathode, the anode, and the electrolyte. In electrolytic cells (as is the case in most electrochemical cells), oxidation occurs at the anode and reduction occurs at the cathode.
What is an Electrolytic Cell?
An electrolytic cell can be defined as an electrochemical device that uses electrical energy to facilitate a non-spontaneous redox reaction. Electrolytic cells are electrochemical cells that can be used for the electrolysis of certain compounds. For example, water can be subjected to electrolysis (with the help of an electrolytic cell) to form gaseous oxygen and gaseous hydrogen. This is done by using the flow of electrons (into the reaction environment) to overcome the activation energy barrier of the non-spontaneous redox reaction.
What is the purpose of the flow of electrons in an electrolytic cell?
This is done by using the flow of electrons (into the reaction environment) to overcome the activation energy barrier of the non-spontaneous redox reaction. The three primary components of electrolytic cells are: The electrolyte provides the medium for the exchange of electrons between the cathode and the anode.
Which cell is negatively charged?
In electrolytic cells, the cathode is negatively charged and the anode is positively charged. The positively charged ions flow towards the cathode whereas the negatively charged ions flow towards the anode.
What is cell notation?
Cell notations are a shorthand description of voltaic or galvanic (spontaneous) cells. The reaction conditions (pressure, temperature, concentration, etc.), the anode, the cathode, and the electrode components are all described in this unique shorthand. Recall that oxidation takes place at the anode and reduction takes place at the cathode.
Which half cell is described first?
The anode half-cell is described first; the cathode half-cell follows. Within a given half-cell, the reactants are specified first and the products last. The description of the oxidation reaction is first, and the reduction reaction is last; when you read it, your eyes move in the direction of electron flow. Spectator ions are not included.
How are the anode and cathode separated?
The cell anode and cathode (half-cells) are separated by two bars or slashes, which represent a salt bridge. The anode is placed on the left and the cathode is placed on the right. Individual solid, liquid, or aqueous phases within each half-cell are written separated by a single bar.
Where to write concentrations of dissolved species?
Concentrations of dissolved species can be written in the parentheses after the phase notation (s, l, g, or aq).
What is the difference between a positive and negative cell potential?
A positive cell potential indicates that the reaction proceeds spontaneously in the direction in which the reaction is written . Conversely, a reaction with a negative cell potential proceeds spontaneously in the reverse direction.
