How do you draw molecular orbital energy diagrams?
FUNDAMENTAL STEPS IN DERIVING MO DIAGRAMSFind the valence electron configuration of each atom in the molecule. ... Decide if the molecule is homonuclear of heteronuclear. ... Fill molecular orbitals using energy and bonding properties of the overlapping atomic orbitals. ... Use the diagram to predict properties of the molecule.
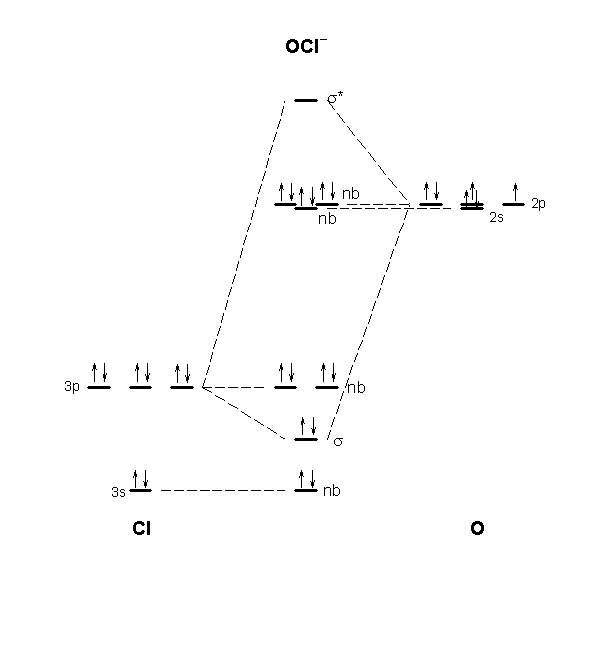
What is a molecular orbital energy level diagram?
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
How do you draw MOS?
1:0620:22How to Draw Molecular Orbital Diagrams (MO ... - YouTubeYouTubeStart of suggested clipEnd of suggested clipNow the thing with mo diagrams is that we have to have sp2 hybridized atoms brand in order for us toMoreNow the thing with mo diagrams is that we have to have sp2 hybridized atoms brand in order for us to draw. Show some sort of bonding. Which has to do with pi electrons.
How do you draw a molecular orbital diagram for organic compounds?
11:5024:19Molecular orbital (MO) diagrams in organic chemistry - YouTubeYouTubeStart of suggested clipEnd of suggested clipWe always start at the lowest energy. And each molecular orbital can hold two electrons. So we'reMoreWe always start at the lowest energy. And each molecular orbital can hold two electrons. So we're designating electrons by drawing an arrow here.
How do you draw a molecular structure?
7:1612:45A Brief Introduction to Drawing Molecules - YouTubeYouTubeStart of suggested clipEnd of suggested clipNotice that the interior carbon is depicted as an angle. And the two terminal carbons are of courseMoreNotice that the interior carbon is depicted as an angle. And the two terminal carbons are of course the term and I of my structure here so this is my line angle formula for propane.
What does energy level diagram mean?
An energy level diagram illustrates the various discrete energy levels (or, energy states) for an atom, ion or molecule. An energy level diagram may include only electronic states, only vibrational states or both electronic and vibrational states.
What is atomic orbital and molecular orbital?
The major difference between atomic and molecular orbitals is that atomic orbitals represent electron density in space associated with a particular atom. Molecular orbitals are associated with the entire molecule, meaning the electron density is delocalized (spread out) over more than one atom.