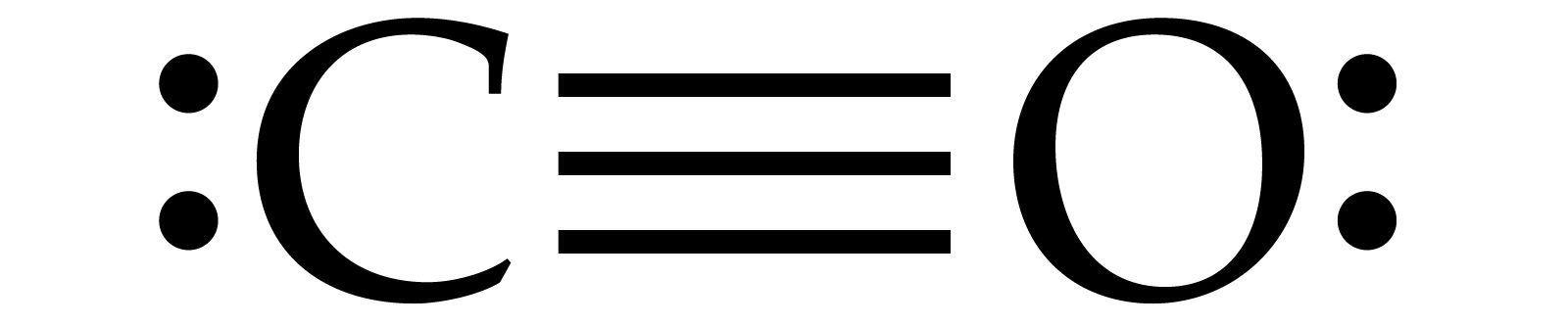
Lewis Structure for CO2 can be drawn by placing Carbon atom as center atom and forming double covalent bond between both oxygen atoms and carbon atom. Also you need to place 4 lone pairs on oxygen atoms 2 by 2. How to draw lewis structure for CO2?
How to create Lewis structures?
To generate the Lewis dot structure, you have to follow the given steps:
- Find the total count of valence electrons to molecules. ...
- Find the required count of electrons needed to make the atoms complete.
- After then, define the number of bonds in the given molecule.
- Later on, choose a central atom.
- And then, draw a skeletal figure.
- After completion of the skeletal figure, place electrons around outside atoms to complete it.
What is the Lewis dot diagram of CO2?
CO2 lewis structure contains two oxygen atoms and one carbon atom, connected with the double bond whereas carbon is the central atom, and no lone pair is present on it. But each oxygen in the CO2 lewis dot structure has two lone pairs. A lewis diagram helps us to know how electrons are arranged around individual atoms in a molecule.
How to make Lewis dot structure?
Method 2 Method 2 of 3: Creating Lewis Structures for Larger Covalent Molecules Download Article
- Determine which atom is your central atom. This atom is usually least electronegative. ...
- Consider the valence electrons of the central atom. As a general (but not all-exclusive) rule, atoms like to be surrounded by 8 valence electrons (the octet rule).
- Write the symbol of your central atom. ...
- Show the electron geometry of the central atom. ...
What is the Lewis dot structure of carbon?
Lewis Dot Structure Definition. Lewis dot structure definition: a visual way to clearly depict the connection of atoms and the electrons present in a molecule.With a carbon Lewis dot structure, one can see how the atoms in a molecule are bonded together, which gives us more information about the structure than the molecular formula.
How do you draw the molecular structure of CO2?
0:322:28Molecular Structure of CO2 (Carbon Dioxide) - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo let's just put our carbon in the center when we draw structures lewis structures we put the leastMoreSo let's just put our carbon in the center when we draw structures lewis structures we put the least electronegative element in the center that's the carbon with the oxygens. On either side.
How many Lewis structures are there for CO2?
three resonating structuresCO2, has three resonating structures, out of which one is a major contributing structure .
What is the structure of CO2?
Carbon dioxide molecules consist of one carbon atom and two oxygen atoms. It has two double bonds between the carbon and oxygen atom. Each double bond is made of one sigma and one pi bond. In all the carbon dioxide molecule contains two sigma and two pi bonds.
How do you draw Lewis structures?
How to Draw a Lewis StructureStep 1: Find the Total Number of Valence Electrons. ... Step 2: Find the Number of Electrons Needed to Make the Atoms "Happy" ... Step 3: Determine the Number of Bonds in the Molecule. ... Step 4: Choose a Central Atom. ... Step 5: Draw a Skeletal Structure. ... Step 6: Place Electrons Around Outside Atoms.More items...•
What is the correct Lewis structure for CO2 quizlet?
21) The correct Lewis structure for CO2 shows that the carbon atom has two sets of lone pair electrons. 22) When calculating the number of electrons for the Lewis structure of a polyatomic ion, you must add one electron for each negative charge and subtract one electron for each positive charge.
What is the Lewis structure of CO2 and what its molecular geometry?
CO2 has 2 electron domains, resulting in a linear electron domain geometry. Both electron domains are bonding pairs, so CO2 has a linear molecular geometry with a bond angle of 180°.
Why is CO2 a Lewis acid?
Acids and Bases: Lewis Theory Carbon dioxide is a polar molecule whose positive center is on the carbon atom: This positive center is able to attract (and accept) the lone electron pairs present on the oxide ion (O2-). Thus, carbon dioxide is acting as a Lewis acid and the oxide ion is acting as a Lewis base.
What is the shape of CO2?
linearCarbon dioxide is linear, while sulphur dioxide is bent (V-shaped). In the carbon dioxide, the two double bonds try to get as far apart as possible, and so the molecule is linear.
How many lone pairs are in CO2?
How many lone pairs are there in the Lewis structure CO2? Each oxygen atom in the CO2 molecule has two lone pairs of electrons.
How many bonds are in CO2?
Carbon dioxide contains two double bonds. Each double bond is comprised of one sigma bond and one π bond.
CO2 Lewis Dot Structure
When drawing the Lewis structure of the carbon dioxide molecule, the carbon and an unpaired electron of oxygen share with each other. As a result, a single covalent bond between carbon and oxygen occurs. However, in this case, carbon and oxygen cannot complete the octet.
What is CO2 and What Does It Do?
CO2, or carbon dioxide, is a compound formed by a covalent bond between one carbon and two oxygen atoms. Generally, living things that survive through the respiratory tract take oxygen back into their lungs as carbon dioxide. Carbon dioxide, which is among the most important components for life, also has many functions.
What Does CO2 Symbolize and Where Is It Used?
If the question of what is the symbol of Co2 will be answered, it can be said that it is carbon dioxide. It is formed as a result of the chemical combination of two atoms naturally bonded with an electron, known as a covalent bond. It is observed that sometimes people, animals and sometimes various reactions occur in nature.
How to draw a Lewis structure of CO2?
The first step is to sketch the Lewis structure of the CO2 molecule, to add valence electrons around the carbon atom; the second step is to add valence electrons to the two oxygen atoms, and the final step is to combine the step1 and step2 to get the CO2 Lewis Structure.
How to calculate the formal charge on oxygen and carbon atoms in CO2 Lewis Structure?
In the following computation, the formal charge will be calculated on the central carbon atom of the CO2 Lewis dot structure.
How many valence electrons does carbon have?
Carbon has four outermost valence electrons, indicating that it possesses four electrons in its outermost shell, whereas oxygen has six valence electrons in its outermost shell. To complete the octet of the oxygen atom requires two valence electrons on each of their outermost shell.
What is the relationship between oxygen and carbon?
Two oxygen atoms establish covalent connections with the central carbon atom as a result, leaving the carbon atom with no lone pairs. There are no lone pairs of electrons on the carbon central atom that resists the bond pairs of the two C-O bonds. According to VSEPR theory, the single C-O bond pairs polarity leads the CO2 molecule to take on the linear geometry structure.
Why is CO2 a linear molecular shape?
The CO2 molecule has a linear molecular geometry because there is no electrical repulsion between the lone pairs of electrons in oxygen and two double bond pairs (C-O) of the CO2 molecule.
What is the chemical formula for CO2?
The carbon dioxide chemical formula is CO2. Drawing CO2 Lewis Structure is very easy to by using the following method. Here in this post, we described step by step method to construct CO2 Lewis Structure. The oxygen and carbon elements come as the member of the oxygen and carbon family groups from the periodic table respectively. The valence electrons in oxygen and carbon are six and one respectively. Carbon dioxide is used as a nonpolar gas molecule. It is essential for photosynthesis in plants.
Why does CO2 have a zero dipole moment?
As a result, it has the zero dipole moment. The CO2 molecule has a zero dipole moment due to an equal charge distribution of negative and positive charges. But both oxygen and carbon atoms fall on the oxygen and carbon family groups in the periodic table respectively. The oxygen atom is a lightly higher electronegative value than carbon in the CO2 molecule. The CO2 molecule has the net dipole moment of 0D value in the ground state energy level.
