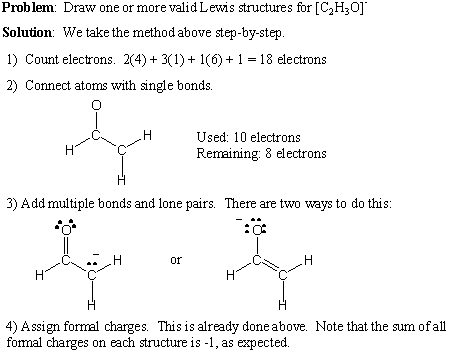What are the five steps for drawing a Lewis diagram for a covalent compound? How to Draw a Lewis Structure Step 1: Find the Total Number of Valence Electrons. Step 2: Find the Number of Electrons Needed to Make the Atoms “Happy” Step 3: Determine the Number of Bonds in the Molecule.
How do you draw a Lewis Diagram of an atom?
Step 1. Determine the total number of valence electrons to be depicted in the Lewis diagram. Step 2. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Step 3. Determine how many electrons must be added to central element.
What is the Lewis dot structure for a single atom?
A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion. The periodic table has all of the information needed to draw a Lewis dot structure. Each Group, or column, is indicated by a roman numeral which represents the number of valence electrons.
How do you write a triple bond in Lewis structure?
A triple bond forms when three electron pairs are shared by a pair of atoms, as in carbon monoxide (CO) and the cyanide ion (CN – ): For very simple molecules and molecular ions, we can write the Lewis structures by merely pairing up the unpaired electrons on the constituent atoms.
How do you find the valence electrons of an atom?
Using Lewis Dot Structures to Show Valence Electrons Lewis dot structures can be drawn to show the valence electrons that surround an atom itself. This type of Lewis dot structure is represented by an atomic symbol and a series of dots. See the following examples for how to draw Lewis dot structures for common atoms involved in covalent bonding.
How to construct Lewis electron structures?
1. Determine the total number of valence electrons in the molecule or ion. Add together the valence electrons from each atom. (Recall that the number of valence electrons is indicated by the position of the element in the periodic table.) 2.
Which compounds have covalent bonds?
The most common examples are the covalent compounds of beryllium and boron. For example, beryllium can form two covalent bonds, resulting in only four electrons in its valence shell: Boron commonly makes only three covalent bonds, resulting in only six valence electrons around the B atom. A well-known example is BF 3:
How many electrons are used in a bonding pair?
Placing a bonding pair of electrons between each pair of bonded atoms gives the following: 6 electrons are used, and 6 are left over. 4. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen).
How many lone pairs of electrons are in a molecule of Cl?
These electrons will usually be lone pairs. For example with Cl, it will have three lone pairs, and one bond if the molecule CCl 4. 5.
What is the measure of the relative attraction for the pair of electrons in a bond?
Arrange the atoms to show specific bonds. When there is a central atom, it is usually the least electronegative element in the compound. Electronegativity is a measure of the relative attraction for the pair of electrons in a bond. See section 4.2 for more details and a table of electronegativity values.
How many electrons do you add to each atom to make an octet?
4. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen).
How to give carbon an octet of electrons?
To give carbon an octet of electrons, we use one of the lone pairs of electrons on oxygen to form a carbon–oxygen double bond:
How to give carbon an octet of electrons?
To give carbon an octet of electrons, we use one of the lone pairs of electrons on oxygen to form a carbon–oxygen double bond:
Why do we use Lewis dot symbols?
Lewis dot symbols provide a simple rationalization of why elements form compounds with the observed stoichiometries. A plot of the overall energy of a covalent bond as a function of internuclear distance is identical to a plot of an ionic pair because both result from attractive and repulsive forces between charged entities. In Lewis electron structures, we encounter bonding pairs, which are shared by two atoms, and lone pairs, which are not shared between atoms. Lewis structures for polyatomic ions follow the same rules as those for other covalent compounds. There are three violations to the octet rule: odd-electron molecules, electron-deficient molecules, and expanded valence shell molecules.
What is the name of the group of atoms that are covalently bonded together and carry an overall electrical?
Recall that a polyatomic ion is a group of atoms that are covalently bonded together and which carry an overall electrical charge. The ammonium ion, NH 4 +, is formed when a hydrogen ion ( H +) attaches to the lone pair of an ammonia ( NH 3) molecule in a coordinate covalent bond.
How many electrons do you add to each atom to make an octet?
4. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen).
What is the central atom of a compound?
When there is a central atom, it is usually the least electronegative element in the compound. Chemists usually list this central atom first in the chemical formula (as in CCl 4 and CO 32−, which both have C as the central atom), which is another clue to the compound’s structure.
How many electrons can an atom hold?
We will explain later that some atoms are able to accommodate more than eight electrons.
Why is C the central atom?
Because carbon is less electronegative than oxygen and hydrogen is normally terminal, C must be the central atom.
What do you use to show covalent bonding in PCL 3?
Use a Lewis electron dot diagram to show the covalent bonding in PCl 3.
Why are Lewis structures important?
Lewis Structures are helpful because they are models that connect well with experimental data. They are symbolic representations that reveal structure on the microscopic level. But these models are only as good as their connection to macroscopic descriptions, based on observations of real substances.
How many unpaired electrons does NH3 have?
The N atom has the following Lewis electron dot diagram: It has three unpaired electrons, each of which can make a covalent bond by sharing electrons with an H atom. The electron dot diagram of NH3 is as follows:
How many electrons does fluorine have?
As another example, consider fluorine. F atoms have seven electrons in their valence shell: These two atoms can do the same thing that the H atoms did; they share their unpaired electrons to make a covalent bond.
Why is the H atom a diatomic molecule?
Because each H atom has a filled valence shell, this bond is stable, and we have made a diatomic hydrogen molecule. It is common to represent the covalent bond with a dash connecting the two elemental symbols, instead of with two dots:
How many atoms can form a covalent bond?
More than two atoms can participate in covalent bonding, although any given covalent bond will be between two atoms only. Consider H and O atoms: The H and O atoms can share an electron to form a covalent bond:
When do atoms share electrons?
As previously mentioned, when a pair of atoms shares one pair of electrons, we call this a single bond. However, a pair of atoms may need to share more than one pair of electrons in order to achieve an octet. A double bond forms when two pairs of electrons are shared between a pair of atoms, as between the carbon and oxygen atoms in CH 2 O (formaldehyde) and between the two carbon atoms in C 2 H 4 (ethylene):
What is Lewis dot structure?
The Lewis Dot Structure is a visual which represents the outermost shell of electrons, also known as valence electrons, and possible covalent bonds within an atom or molecule. These valence electrons are negatively charged and are attracted to the positively charged nucleus, made up of neutrons and protons.
How many electrons can oxygen fit in a Lewis dot?
Draw the Lewis Dot Structure for Oxygen. Since Oxygen is in Period 2, it can fit a maximum of eight (8) electrons second energy level. Oxygen Group VI, which means it has a total of six (6) valence electrons around the atom.
How to determine if a molecule has more than one element?
If a molecule has more than one element, add the valence electron of all elements present in the compound. Determine which atom will be the central atom of the Lewis Dot Structure. The central atom is the least most electronegative atom in the compound. Remember the trend for electronegativity on the periodic table.
What are the particles that are smaller than atoms?
The only thing smaller than atoms are their subatomic particles; electrons, protons, and neutrons. Not even under a complex microscopic can we view the individual electrons that surround an atom’s nuclei. The Lewis Dot Structure is a visual which represents the outermost shell of electrons, also known as valence electrons, and possible covalent bonds within an atom or molecule. These valence electrons are negatively charged and are attracted to the positively charged nucleus, made up of neutrons and protons. Keep in mind that in reality electrons are constantly moving around the nucleus and are not rooted in one place as portrayed in a 2D structure.
How many valence electrons are in a nitrogen and hydrogen bond?
The total valence electrons of the nitrogen and four hydrogens is 9 electrons. Since there is a positive charge of 1+ that means there is one less electron, so there will be a total of 8, which are represented by the four bonds as lines. Example 3. OH –, Hydroxide ion.
How many valence electrons are in Group II?
All elements in Group II have two (2) valence electrons, all the way up to VIII, eight (8) valence electrons. Properties are also consistent across the rows, or periods, of the periodic table. Periods are indicated by a number, 1, 2, 3, etc. which represent the energy level, or shell of electrons.
How many electrons does phosphorus hold?
There are a few things to keep in mind. Phosphorus is in Period 3, which means it can hold more than 8 electrons and creates a double bond to the oxygen which fulfills the octet rule for one oxygen, but not the others.