
Following are the significant figures rules that govern the determination of significant figures:
- Those digits which are non-zero are significant. ...
- If any zero precedes the non-zero digit then it is not significant. ...
- If there is a zero between two non-zero digits then it is also a significant figure. ...
- Zeroes at the end or on the right side of the number are also significant. ...
- START counting for sig. figs. On the FIRST non-zero digit.
- STOP counting for sig. figs. On the LAST non-zero digit.
- Non-zero digits are ALWAYS significant.
- Zeroes in between two non-zero digits are significant. All other zeroes are insignificant.
How to count significant figures in chemistry?
Tips and Rules for Determining Significant Figures
- Significant Figure Rules. Non-zero digits are always significant. ...
- Uncertainty in Calculations. Measured quantities are often used in calculations. ...
- Losing Significant Figures. Sometimes significant figures are 'lost' while performing calculations. ...
- Rounding and Truncating Numbers. ...
- Exact Numbers. ...
- Accuracy and Precision. ...
- Sources. ...
What are the significant figures in chemistry?
Significant figures in chemistry reflect the accuracy and precision of the experimental process being used. In general, the quantitative results obtained from the use of several measuring devices having varying degrees of accuracy should be expressed in terms of the device having the lowest degree of accuracy.
What is the definition of significant figures in chemistry?
What are the Significant Figures in Chemistry | Examples
- Definition of Significant Figures. In chemistry, Significant figures are the digits of value which carry meaning towards the resolution of the measurement.
- Examples of Significant Figures
- Digits which are not the Significant Figure. All leading zeros. ...
- Rules of Significant Figures. ...
- Overall Examples of Significant Figures. ...
What are the 5 rules for significant figures?
Significant Figures All non-zero numbers ARE significant. ... Zeros between two non-zero digits ARE significant. ... Leading zeros are NOT significant. ... Trailing zeros to the right of the decimal ARE significant. ... Trailing zeros in a whole number with the decimal shown ARE significant. . Discover more science & math facts & informations.
How to determine how many significant digits are present?
1. All non-zero numbers are significant. For example, the number 843 would have three significant digits are they are all non-zero. 2. Zeros between non-zero numbers are significant.
Why Significant Digits?
Significant digits (also called significant figures or “sig figs” for short) indicate the precision of a measurement. A number with more significant digits is more precise. For example, 8.00 cm is more precise than 8.0 cm.
What does 250 mean in decimal?
250. – The decimal point indicates the measurement is precisely 250. not around 250 as in the previous example. This number would have three significant digits.
Why are the two zeros between 7 and 8 significant?
The two zeros between 7 and 8 are significant because they are between non-zero numbers (see rule #2 above). The trailing zero is significant because the number contains a decimal place (see rule #4 above).
When recording a measurement, do we include all of the known digits plus a final estimated digit?
It’s sometimes easier to think of this in terms of recording all of the known “places” (ones , tenths, hundredths) plus a final estimated place. For example, in the first ruler below it is marked every one centimeter so we know the ones place and could record 2. But we must also estimate one, and only one, additional digit. So in this case we might record 2.3 cm or perhaps 2.4 cm. Either would be correct as the 2 (ones place) is precisely known while the final digit (tenths place) is estimated.
What is the significance of 0.0025?
In the number 0.0025, the leading zeros (those to the left of the non-zero numbers) are not significant. Therefore, only the 2 & 5 are counted meaning it has two significant digits. The leading zeros are known as placeholder zeros as they do not add to the precision of the measurement, they simply occupy the ones, tenths, and hundredths places. This will become more clear in our lesson on Scientific Notation as 0.0025 could also be written as 2.5×10 -3, completely eliminating the leading zeros.
What is significant at the end of a number?
Zeroes at the end of a number are significant if there is a decimal point.
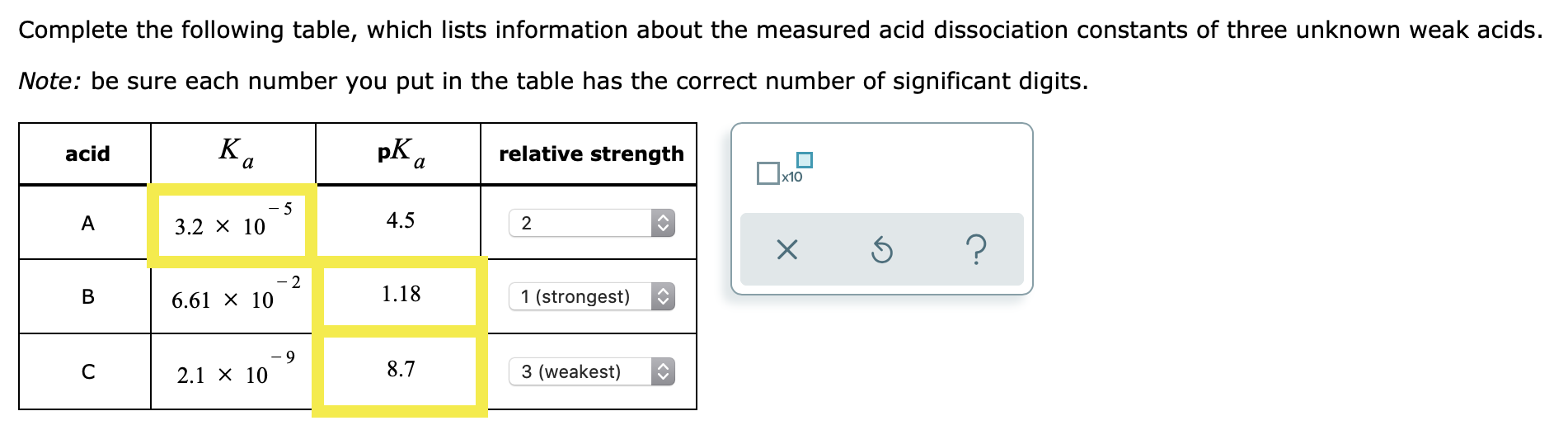
How many significant figures are there in the number 2.30 x 10 5?
The number 2.30 x 10 5 has 3 significant figures because its coefficient 2.30 has 3 sig figs.
How many sig figs are there in 0.081?
Here, the calculation involves multiplication and division, so the answer has the same sig figs as the number with the least number of sig figs. The numbers 0.081, 1090 and 31.0 have 2, 4 and 3 sig figs, respectively. The answer should have only 2 sig figs. The calculator’s answer is 2.848064516. We need to round off the answer to 2.8.
Why are all digits of intermediate answers carried through in the calculation?
All digits of intermediate answers are carried through in the calculation while noting how many significant figures or decimal places they really should have. This minimizes deviations from the final answer that occurs when intermediate answers are estimated.
What is the decimal place of 12.387?
The calculator gives the answer 12.387. We’re adding here, so the rule of least number of decimal places applies. One number has 3 decimal places, and the other has 1 decimal place. The answer should, therefore, have 1 decimal place, so the answer is 12.4.
How to express certainty of a value?
Another way of expressing the certainty of a value is the number is significant figures, or sig figs, for short. In the example above, $70 and $78 both have no decimal place, but $78 is more certain because it has more significant figures than $70. Counting the significant figures in a number is one of the first topics covered in a Chemistry ...
What is the first topic covered in a chemistry class?
Counting the significant figures in a number is one of the first topics covered in a Chemistry or Physics class. Many students can get stumped by sig figs, and worse, teachers dock off points for wrong sig figs in homework, exams and lab reports. You need to figure out the deal with sig figs as soon as possible, and we’re here to help!
What is the first significant digit?
The first three significant digits are 17.8. The first nonsignificant digit is 5. According to rule #2, because it is 5 or greater then we add 1 to the last significant digit (the 8 becomes a 9) and drop all of the nonsignificant digits. The answer is thus 17.9.
How many nonsignificant digits are retained in a calculation?
If a calculation has two or more operations, retain at least one nonsignificant digit until the final operation and then round off the answer.
How to get rid of nonsignificant digits?
However, we often generate nonsignificant digits when performing calculations. We get rid of nonsignificant digits by rounding off numbers. There are three rules for rounding off numbers.
How many significant digits are in 151 ml?
Round the measurement 151 mL to 2 significant digits.
What are the rules for rounding numbers?
Rules for Rounding Numbers. If the first nonsignificant digit is less than 5 , drop all nonsignificant digits. If the first nonsignificant digit is greater than or equal to 5, increase the last significant digit by 1 and drop all nonsignificant digits. *. If a calculation has two or more operations, retain at least one nonsignificant digit until ...
What is significant figure in chemistry?
Significant figures in chemistry reflect the accuracy and precision of the experimental process being used. In general, the quantitative results obtained from the use of several measuring devices having varying degrees of accuracy should be expressed in terms of the device having the lowest degree of accuracy. Such establishes reasonable expectations for data reproducibility using a specified experimental process. An excellent 10 minute video on counting and applying significant figures is found at
How many significant digits are in 0.1024?
EX: 0.1024 −5293, or 0.10245293, is stated to only have 4 significant digits.
How many zeros are in 2000?
EX: 2000. has 3 significant zeroes, although it is better to write this as 2.000 × 103, scientific notation.
Does 007 have two leading zeros?
EX: 007 has two leading zeroes that do not matter. We could just say 7 and it numerically says the same thing.
What is the most significant digit?
The leftmost non-zero digit is sometimes called the most significant digit or the most significant figure. For example, in the number 0.004205, the '4' is the most significant figure. The left-hand '0's are not significant. The zero between the '2' and the '5' is significant. The rightmost digit of a decimal number is the least significant digit ...
What is the rightmost digit of a decimal?
The rightmost digit of a decimal number is the least significant digit or least significant figure. Another way to look at the least significant figure is to consider it to be the rightmost digit when the number is written in scientific notation. Least significant figures are still significant!
How to distinguish accuracy and precision?
Arrows surrounding a bullseye indicate a high degree of accuracy; arrows very near to each other (possibly nowhere near the bullseye) indicate a high degree of precision. To be accurate, an arrow must be near the target; to be precise successive arrows must be near each other. Consistently hitting the very center of the bullseye indicates both accuracy and precision.
What is the least significant number in 5800?
If no decimal point is present, the rightmost non-zero digit is the least significant figure. In the number 5800, the least significant figure is '8'.
Why is there uncertainty in measurement?
Every measurement has a degree of uncertainty associated with it. The uncertainty derives from the measuring device and the skill of the person doing the measuring. Scientists report measurements using significant figures to reflect this uncertainty.
Do pure numbers affect accuracy?
This is true when using defined quantities, including many conversion factors, and when using pure numbers. Pure or defined numbers do not affect the accuracy of a calculation. You may think of them as having an infinite number of significant figures. Pure numbers are easy to spot because they have no units.
How to determine if a number is significant?
There are three rules on determining how many significant figures are in a number: 1 Non-zero digits are always significant. 2 Any zeros between two significant digits are significant. 3 A final zero or trailing zeros in the decimal portion ONLY are significant.
What is the number of significant figures in multiplication?
This means that if you multiplied or divided three numbers: 2.1, 4.005 and 4.5654 , the value 2.1 which has the fewest number of digits would mandate that the answer be given only to two significant figures.
How to convert a small number to an exponent?
You simply move the decimal to the right until only one non-zero digit is in front of the decimal point. The exponent then equals the number of digits you had to pass along the way.
What is a significant zero?
Any zeros between two significant digits are significant.
Why do we use scientific notation?
Another reason we often use scientific notation is to accommodate the need to maintain the appropriate number of significant figures in our calculations.
What is an exact number?
Exact Numbers. Exact numbers, such as the number of people in a room, have an infinite number of significant figures. Exact numbers are counting up how many of something are present, they are not measurements made with instruments. Another example of this are defined numbers, such as.
How to convert decimals to exponential numbers?
To convert this to an exponential number we need to move the decimal to the left until only one digit resides in front of the decimal point. In this number we move the decimal point 5 times.
How to find the number of significant figures?from thoughtco.com
The number of significant figures is determined by starting with the leftmost non-zero digit. The leftmost non-zero digit is sometimes called the most significant digit or the most significant figure. For example, in the number 0.004205, the '4' is the most significant figure. The left-hand '0's are not significant.
How many significant digits are in 108.0097?from byjus.com
For example, 108.0097 contains seven significant digits. All zeros that are on the right of a decimal point and also to the left of a non-zero digit is never significant. For example, 0.00798 contained three significant digits. All zeros that are on the right of a decimal point are significant, only if, a non-zero digit does not follow them.
What happens if the digit next to the rounding off digit is greater than 5?from byjus.com
But if the digit next to the rounding off digit is greater than 5, then we have to add 1 to the rounding off digit and exclude the other numbers on the right side.
Which zeros are significant?from byjus.com
All the zeros that are on the right of the last non-zero digit are significant if they come from a measurement. For example, 1090 m contains four significant digits.
How to distinguish accuracy and precision?from thoughtco.com
Arrows surrounding a bullseye indicate a high degree of accuracy; arrows very near to each other (possibly nowhere near the bullseye) indicate a high degree of precision. To be accurate, an arrow must be near the target; to be precise successive arrows must be near each other. Consistently hitting the very center of the bullseye indicates both accuracy and precision.
What is the least significant number in 5800?from thoughtco.com
If no decimal point is present, the rightmost non-zero digit is the least significant figure. In the number 5800, the least significant figure is '8'.
When is the last digit rounded up?from byjus.com
When the first digit in left is less than 5, the last digit held should remain constant. When the first digit is greater than 5, the last digit is rounded up. When the digit left is exactly 5, the number held is rounded up or down to receive an even number.
Why Significant digits?
Recording Measurements
- When recording a measurement we include all of the known digits plus a final estimated digit. It’s sometimes easier to think of this in terms of recording all of the known “places” (ones, tenths, hundredths) plus a final estimated place. For example, in the first ruler below it is marked every one centimeter so we know the ones place and could record 2. But we must also estimate one, a…
Counting Significant Digits
- If a measurement has already been made and provided, then we can determine how many significant digits are present by following a few simple rules. 1. All non-zero numbers are significant. For example, the number 843 would have three significant digits are they are all non-zero. 2. Zeros between non-zero numbers are significant. The number 307 woul...
Counting Significant Digits Examples
- 1. 23.5– Three significant digits as all are non-zero numbers (see rule #1 above). 2. 23.50– Four significant digits. The final zero is significant because the number contains a decimal place (see rule #4 above). 3. 402– Three significant digits. The zero is between non-zero numbers (see rule #2 above). 4. 5,200– Two significant digits. There is no decimal place so the trailing zeros are si…
How to Count Significant Figures
- Rule 1 (for non-zeroes):
All non-zero digits are significant. This rule should be simple, if the digit is not zero, count it as significant. - Rule 2 (for sandwiched zeroes):
Zeroes sandwiched between two significant digits are significant.
Numbers in Scientific Notation
- For scientific notation, count the sig figs for the coefficient. In counting the sig figs for the number 8.06 x 10-3, consider only the coefficient 8.06 when counting, so there are 3 significant figures. The number 2.30 x 105has 3 significant figures because its coefficient 2.30 has 3 sig figs.
Exact Numbers
- Numbers that are obtained by measurement are called inexact numbers since there is always a degree of uncertainty about the measured value. The mass of the piece of mail measured by a lab scale at 14.46 g comes with an error of ±0.01, and its value can be anywhere between 14.45 g to 14.47 g. Exact numbers are numbers that are known with complete certainty. Values obtained b…
How to Use Significant Figures in Calculations
- Image Source: Flickr When adding or subtracting numbers, the answer must be expressed with the same number of decimal places as the participating number with the least number of decimal places. When multiplying or dividing numbers, the answer must be expressed with the same number of significant figures as the participating number with the least number of significant fig…