How to Calculate Atomic Mass?
- 1. By referring to the periodic table In the periodic table digit of an atomic mass usually marked under the representation of an element. For example ...
- 2. Addition Of Mass Of Protons and Neutrons In an element atomic mass of an atom can be calculated by adding the mass of protons and neutrons.
- 3. All Atoms of an Element – Weighted Average
What is the formula for calculating atomic mass?
Atomic mass = Number of protons + number of neutrons + number of electrons In other words, when proton, electrons, and neutrons of one atom are added together then this is named as average mass of the atom.
What information does the atomic mass tell you?
This atomic number tells you the the number of protons and electrons in an atom . What does the atomic mass tell you about the atom of that element? The atomic mass tells us the weight of protons and neutrons. How do you know how many electrons there are surrounding the nucleus of a particular atom? What does the period number represent?
What is atomic mass and how is it determined?
The atomic mass of the atom is the mass of the protons plus the mass of the neutrons, 6 + 7, or 13. 3) Weighted Average for All Atoms of an Element The atomic mass of an element is a weighted average of all the element's isotopes based on their natural abundance. It is simple to calculate the atomic mass of an element with these steps.
How do you calculate the atomic mass of an atom?
What is the formula for calculating atomic mass?
- The periodic table is used to illustrate this. ...
- The sum of the masses of protons and neutrons The atomic mass of an atom of an element may be estimated by summing the masses of the protons and neutrons ...
- A weighted average of all the atoms of an element

What is the easiest way to find the atomic mass?
To calculate the atomic mass of a single atom of an element, add up the mass of protons and neutrons. Example: Find the atomic mass of an isotope of carbon that has 7 neutrons. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons.
How do you find the atomic number and mass?
0:072:23Understanding Atomic Number and Atomic Mass - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe number that appears below the element symbol is called the atomic mass the mass of an atomMoreThe number that appears below the element symbol is called the atomic mass the mass of an atom depends on the number of protons neutrons.
How is atomic number calculated?
The atomic number of an atom is equal to the number of protons in the nucleus of an atom or the number of electrons in an electrically neutral atom. For example, in a sodium atom, there are 11 electrons and 11 protons. Thus the atomic number of Na atom = number of electrons = number of protons = 11.
What makes up the atomic mass?
The atomic mass of an atom is an empirically measured property, which is equivalent to the sum mass of protons, neutrons, and electrons that make up the atom (with a small adjustment for nuclear binding energy).
How do you find the atomic number and protons and neutrons?
0:062:13Calculating the Protons, Neutrons and Electrons for an Atom - YouTubeYouTubeStart of suggested clipEnd of suggested clipOkay you should be familiar with the two numbers on the periodic table the top one being the massMoreOkay you should be familiar with the two numbers on the periodic table the top one being the mass number which is the number of protons. And neutrons add it together. And then the bottom number being
How do you find the atomic number and period of a group?
Looking it up in the periodic table is the fastest way, because not all periods are equally long, and the groups are not always consecutive. The best I can give you is a piece-wise definition; given a period p and a group g, the atomic number n is given by: p=1, g=1: n=1.
What is atomic number and mass number explain with example?
(i) Atomic number: The atomic number is the total number of protons present in the atom. For example, the atomic number of sodium is 11. It contains 11 protons and 11 electrons. (ii) Mass number: It is the sum of the number of neutrons and the number of protons.
Are atomic number and mass number the same?
The major difference between atomic number and mass number is that the atomic number states the number of protons present in an atom whereas, the mass number indicates the total number of protons and the number of neutrons present in an atom.
How to find the atomic mass of an atom?
Since the combined masses of protons and neutrons account for almost all the mass of the given atom, the atomic mass of the single atom can be calculated by adding the total number of protons and the total number of neutrons of that particular isotope. The number of protons in a given atom is always equal ...
What is the atomic mass of an element?
What is Atomic Mass? Atomic mass can be defined as the total mass of one atom of any given element . The unit of atomic mass is called the unified atomic mass unit (denoted by ‘u’). Most of the atomic mass of a substance is made up of protons and neutrons. Therefore, it is almost equal to its mass number.
How to find the abundance percentage of an isotope?
When the sample is a mixture of isotopes of the given element in varying percentages, the following method can be used: Step 1: Multiply the atomic mass of the isotope with its abundance percentage and divide the result by 100. Step 2: Add the values gained from step 1 for each given isotope in the sample.
What is the relative isotopic mass of an element?
Relative isotopic mass refers to the mass of an isotope of an element when compared to one-twelfth of the mass of the carbon 12 isotope (which is equal to 12). It is also called atomic weight.
How to find the element on the periodic table?
To find the element on the periodic table, the element symbol or its atomic number must be known. Once the required data is attained, it can be compared with the periodic table where the atomic mass of the natural sample in atomic mass units will be provided in decimal figures.
How many protons are in an atom?
The number of protons in a given atom is always equal to its atomic number. For example, the atomic number of oxygen is 8, therefore the total number of protons in an oxygen atom is 8. The total number of neutrons is generally specified when describing which isotope the atom belongs to.
Why is the atomic mass of an atom calculated?
The atomic masses for individual atoms must be calculated by taking into account the exact number of protons and neutrons in a single atom.
How to find the atomic number of an element?
Find the atomic number of the element or isotope. The atomic number is the number of protons in an element, and never varies. For example, all hydrogen atoms, and only hydrogen atoms, have 1 proton. Sodium has an atomic number of 11 because its nucleus has 11 protons, while oxygen has an atomic number of 8 because its nucleus has 8 protons. You can find the atomic number of any element on the periodic table - in nearly all standard periodic tables: it's the number above an element's 1 or 2-letter chemical symbol. This number will always be a positive whole number.
How many protons and neutrons are in carbon?
Add the proton and neutron count. This is the atomic mass of that atom. Don't worry about the number of electrons orbiting the nucleus - their combined mass is very, very small, so, in most practical cases, it won't significantly affect your answer. Our carbon atom has 6 protons + 6 neutrons = 12.
Why do chemical elements have different isotopes?
Chemical elements have different isotopes - chemical forms that differ in mass because of the addition or subtraction of one or more neutrons to the atom's nucleus.
How to find molar mass?
However, by simply multiplying an atomic mass by 1 g/mol, a workable quantity is obtained for an element's molar mass - the mass (in grams) of one mole of an element's atoms.
What is the atomic mass of an atom?
Atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule. However, the mass of an electron is so small, it is considered negligible and not included in the calculation. Though technically incorrect, the term is also often used to refer to the average atomic mass of all of the isotopes of one element. This second definition is actually the relative atomic mass, also known as the atomic weight, of an element. The atomic weight takes into account the average of the masses of naturally occurring isotopes of the same element. Chemists need to distinguish between these two types of atomic mass to guide their work - an incorrect value for atomic mass can, for instance, lead to an incorrect calculation of an experiment's yield.
What is the value of an element on the periodic table?
Understand that periodic table values are an average atomic mass for an element. As has been noted, the relative atomic masses listed for each element on the periodic table are average values of all of an atom's isotopes. This average value is valuable for many practical calculations - like, for instance, calculating the molar mass of a molecule comprised of several atoms. However, when dealing with individual atoms, this number is sometimes insufficient.
How to find the mass of a molecule?
Find average molecular mass. Since a molecule is just a collection of atoms, you can add the masses of the atoms together to find the mass of the molecule. If you use the average atomic masses (instead of the mass of a specific isotope), the answer is the average mass of the molecule as found in a naturally occurring sample. Here's an example:
What does average atomic mass tell you?
The average atomic mass tells you the relationship between mass and number of atoms in a typical sample of the element. This is useful in chemistry laboratories because it is almost impossible to count the number of atoms directly, but easy to measure mass.
How many isotopes does silver have?
For example, the element silver (Ag) has two naturally occurring isotopes: Ag-107 and Ag-109 (or 107 Ag and 109 Ag). Isotopes are named after the "mass number," or the sum of protons and neutrons in one atom. This means Ag-109 has two more neutrons per atom than Ag-107, giving it slightly more mass.
What is the mass number of an isotope?
The mass number for each isotope is the sum of numbers of protons and neutrons in the nucleus. Each proton and each neutron weigh 1 atomic mass unit (amu). The only difference between two isotopes of the same element is the number of neutrons per atom, which affects the atom's mass.
Why is it important to know the average atomic mass?
It's important to know average atomic mass because different isotopes of an element exist at different abundances on Earth, so different isotopes contribute to the average atomic mass at different proportions.
Which isotope has more neutrons?
Isotopes with more neutrons have more mass. For example, the silver isotope Ag-107 has an atomic mass of 106.90509 amu (atomic mass units). The isotope Ag-109 is slightly heavier with a mass of 108.90470. The last couple decimal places might be slightly different in different sources.
How to convert atomic mass to molar mass?
Atomic mass units are very small, so chemists typically weigh samples in grams instead. Fortunately, these concepts are defined to make the conversion as easy as possible. Just multiply the average atomic mass by 1 g / mol (the molar mass constant) to get an answer in g / mol instead.
How to find the atomic mass of an atom?
Atomic mass: The atomic mass is the mass of an atom, and is obtained by finding the average mass of the isotopes of a given element.
How much copper has an atomic mass?
Calculate the average atomic mass of copper given that 69.1% of its naturally occurring atoms have a mass of 62.93 amu and 30.9% have a mass of 64.93 amu.
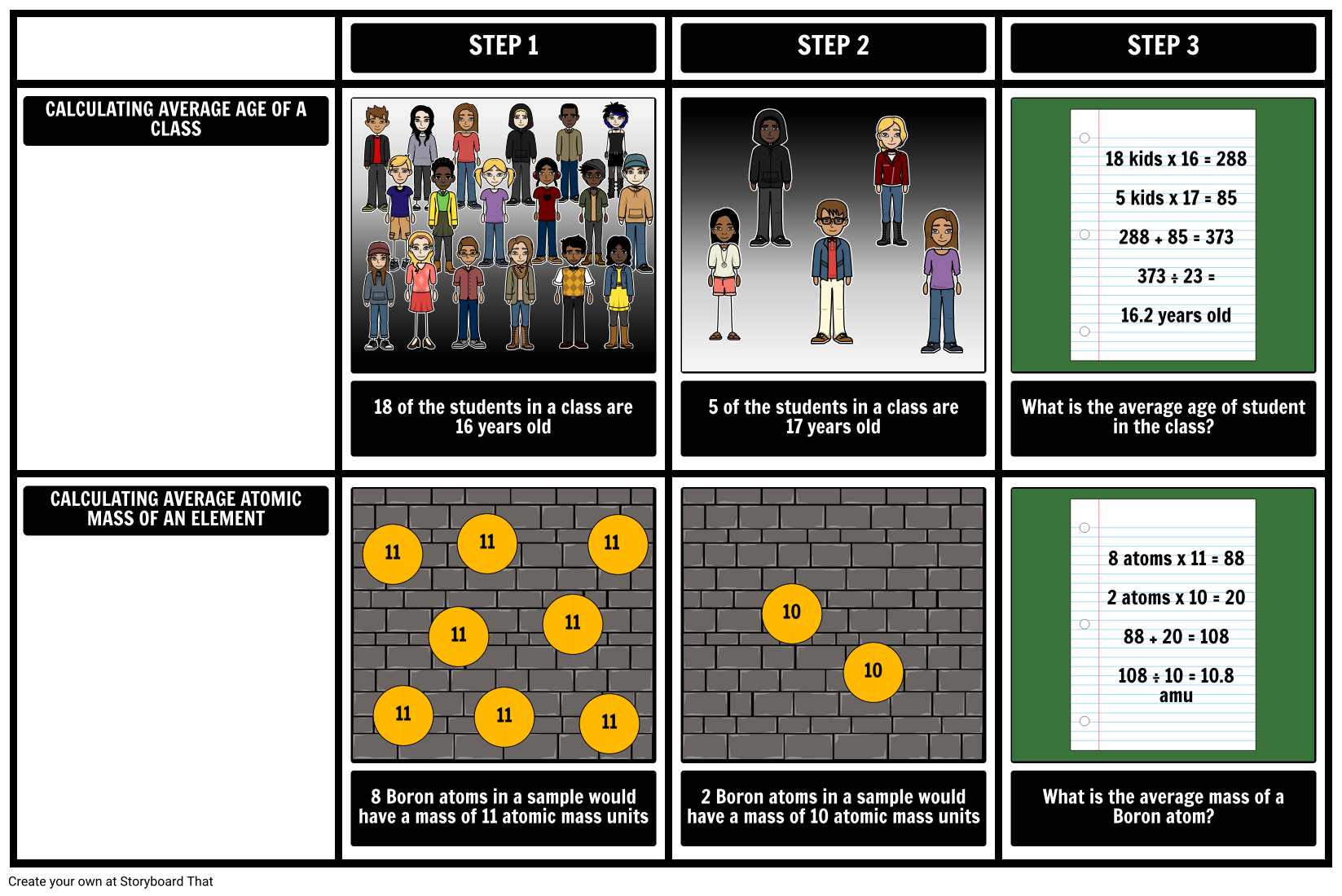
What is the average mass of boron?
Calculate the average atomic mass of boron given that 19.8% of its naturally occurring atoms have a mass of 10.013 amu and 80.2% have a mass of 11.009 amu.
