
How do you find the concentration of glucose?
- Formula to calculate mmol/l from mg/dl: mmol/l = mg/dl / 18.
- Formula to calculate mg/dl from mmol/l: mg/dl = 18 × mmol/l.
How do you determine the concentration of an unknown glucose concentration?
You treat your unknown and a standard set of solutions containing known concentrations of glucose with standard Benedict's solution. You remove the precipitate and measure the intensity of absorption in a colorimeter. A calibration graph prepared from the glucose solutions enables you to determine the concentration of your unknown.
How is the level of glucose measured?
This is an Open Access article distributed under the Creative Commons Attribution Non-Commercial License. The level of glucose in the blood can be measured by applying a drop of blood to a chemically treated, disposable ‘test-strip’, which is then inserted into an electronic blood glucose meter.
How do you find the concentration of a solute?
Be sure to subtract the weight of the container you’re using to measure the solute or else your concentration will be inaccurate. If your solute is a liquid, you may need to calculate the mass using the formula D = m/V, where D is the liquid’s density, m is the mass, and V is the volume.
What is the formula for concentration in chemistry?
In chemistry, a solution’s concentration is how much of a dissolvable substance, known as a solute, is mixed with another substance, called the solvent. The standard formula is C = m/V, where C is the concentration, m is the mass of the solute dissolved, and V is the total volume of the solution.
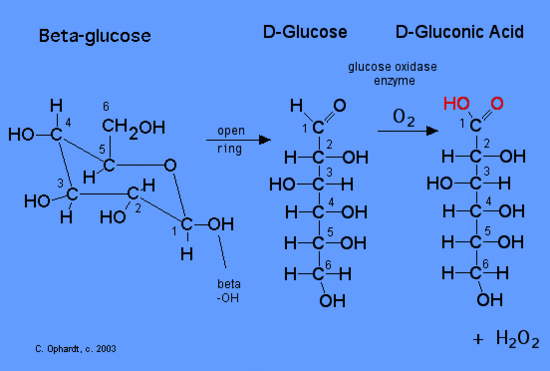
How do you calculate glucose concentration?
The level of glucose in the blood can be measured by applying a drop of blood to a chemically treated, disposable 'test-strip', which is then inserted into an electronic blood glucose meter. The reaction between the test strip and the blood is detected by the meter and displayed in units of mg/dL or mmol/L.
How do you calculate glucose concentration in an unknown solution?
2:388:24Determining Glucose Concentration using a Calibration Curve - YouTubeYouTubeStart of suggested clipEnd of suggested clipAdd two centimeters cubed of each concentration to the corresponding boiling tubes you will alsoMoreAdd two centimeters cubed of each concentration to the corresponding boiling tubes you will also need to add two centimeters cubed of the unknown. Solution. And two centimeters cubed of the distilled.
What is the concentration of glucose in cells?
The amount of glucose in cell culture formulations ranges from 1 g/L (5.5 mM) to as high as 10 g/L (55 mM). Many classical media are supplemented with approximately 5.5 mM D-glucose which approximates normal blood sugar levels in vivo.
How do you find the concentration of glucose solution using colorimetry?
4:035:48A Level Biology Revision "Using a colorimeter to quantify glucose 2"YouTubeStart of suggested clipEnd of suggested clipWe then set the colorimeter to measure absorption. In other words how much red light is absorbed byMoreWe then set the colorimeter to measure absorption. In other words how much red light is absorbed by the solution. We then place a cuvette containing just distilled water into the color emitter.
How do you determine concentration?
Divide the mass of the solute by the total volume of the solution. Write out the equation C = m/V, where m is the mass of the solute and V is the total volume of the solution. Plug in the values you found for the mass and volume, and divide them to find the concentration of your solution.
What is C1V1 C2V2?
C1V1=C2V2 is used to calculate an unknown quantity where two solutions/mixtures are proportional … C1V1 = Concentration/amount (start) and Volume (start) C2V2 = Concentration/amount (final) and Volume (final) 1.
What is the concentration of glucose in the culture medium?
As glucose concentrations relate to cell culture medium, 5.5 mM is considered a low glucose media, while 30 mM is considered high glucose media. Furthermore, media containing 5.5 mM glucose is currently what is most often used and recommended for maintaining hMSCs in culture (36).
What is glucose formula?
C₆H₁₂O₆Glucose / Formula
What is the concentration of glucose when blood rises?
After you've eaten, the concentration of glucose in your blood rises. When it goes too high the pancreas releases insulin into the bloodstream. This insulin stimulates the liver to convert the blood glucose into glycogen for storage.
What was the concentration of glucose in the urine sample?
The normal amount of glucose in urine is 0 to 0.8 mmol/L (millimoles per liter). A higher measurement could be a sign of a health problem. Diabetes is the most common cause of elevated glucose levels.
How do you calculate concentration a level biology?
0:413:57A-Level Biology - Prepare solution with known concentration in % and ...YouTubeStart of suggested clipEnd of suggested clipSolution the first step is to convert the desired percentage into a decimal this conversion is doneMoreSolution the first step is to convert the desired percentage into a decimal this conversion is done by dividing the percentage by 100. When you divide 10 with 100. You will get 0.1.
How do you find the concentration of a calibration curve?
2:074:04A.8.6 Find the concentration of a solution via calibration curve (Beer ...YouTubeStart of suggested clipEnd of suggested clipIt has to be the same chemical of course but with an unknown concentration. If it has an absorptionMoreIt has to be the same chemical of course but with an unknown concentration. If it has an absorption of 1. Then I can use my graph to calculate the concentration. So dotting along dotting.
What happens when glucose is too high?
Glucose concentration is itself the signal and, when too high, increases secretion of a hormone, insulin, which initiates a cascade of events to move glucose to cells and store it as glycogen thereby returning the extracellular glucose concentrations to normal.
How does glucose depletion affect oxygen content?
glucose depletion causes the substrate to decrease, which in turn causes the dissolved oxygen content to oscillate . Therefore, we can indirectly control the glucose concentration by maintaining a constant ethanol concentration and keeping the dissolved oxygen content from oscillating.
What is the easiest way to express the concentration of a solution?
Mass percent composition (also called mass percent or percent composition) is the easiest way to express the concentration of a solution because no unit conversions are required. Simply use a scale to measure the mass of the solute and the final solution and express the ratio as a percentage.
What are the units of concentration?
The most common units are molarity, molality, normality, mass percent, volume percent, and mole fraction. Here are step-by-step directions for calculating concentration, with examples.
How to find moles of KCl?
Start by looking up the number of grams per mole of potassium and chlorine on a periodic table. Then add them together to get the grams per mole for KCl. K = 39.1 g/mol.
What is volume percent?
Volume percent is the volume of solute per volume of solution. This unit is used when mixing together volumes of two solutions to prepare a new solution. When you mix solutions, the volumes aren't always additive, so volume percent is a good way to express concentration.
How to find mass percent?
Calculate Mass Percent: mass solute divided by mass final solution multiplied by 100%.
What is concentration in science?
Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. There are multiple units of concentration. Which unit you use depends on how you intend to use the chemical solution.
How many H+ ions are in sulfuric acid?
You know there are 2 moles of H+ ions (the active chemical species in an acid-base reaction) for every 1 mole of sulfuric acid because of the subscript in the chemical formula. So, a 1 M solution of sulfuric acid would be a 2 N (2 normal) solution.
How to find the concentration of a solution?
To calculate the concentration of a solution, start by converting the solute, or the substance being dissolved, into grams. If you're converting from milliliters, you may need to look up the solute's density and then multiply that by the volume to convert to grams. Next, convert the solvent to liters.
How to find the molar mass of a solute?
Add the atomic masses of the solute together to find the molar mass. Look at the elements in the chemical formula for the solute you’re using. List the atomic mass for each element in the solute since atomic and molar mass are the same. Add together the atomic masses from your solute to find the total molar mass.
What is the solute in chemistry?
The solute is the substance that you’re mixing in to form your solution. If you’re given the mass of the solute in your problem, write it down and be sure to label it with the correct units. If you need to find the mass of the solute, then weigh it on a lab scale and record the measurement.
