If you know that titrating 50.00 ml of an HCl solution requires 25.00 ml of 1.00 M NaOH, you can calculate the concentration of hydrochloric acid, HCl. Based on the molar ratio between HCl and NaOH, you know that at the equivalence point : moles HCl = moles NaOH Acid-Base Titration Solution
- Amount of solute in mol = concentration in mol/dm 3 × volume in dm 3
- Amount of sodium hydroxide = 0.100 × 0.0250.
- = 0.00250 mol.
- The balanced equation is: NaOH(aq) + HCl(aq) → NaCl(aq) + H 2O(l)
- So the mole ratio NaOH:HCl is 1:1.
What is the balanced equation for HCl and NaOH?
Balanced equation for naoh and hcl. This reaction states that 1 molecule of HCl will certainly react v 1 molecule of NaOH to produce 1 molecule the the salt, sodium chloride (NaCl), and 1 molecule of water. The enthalpy change, ΔH 2 was -50.2 kJ/mol. ΔH because that a neutralisation reaction is negative. Compound claims
What is the reaction of acetic acid and NaOH?
The acetic acid (HC2H3O2) found in the vinegar will react with the NaOH until all of the acetic acid is neutralized. When an acid, such as acetic acid reacts with a base like NaOH, the products are a salt (NaC2H3O2, sodium acetate) and water (H2O). This means you have 25.00 g of vinegar in your flask.
What is the concentration of NaOH?
The most concentrated NaOH solution is usually a little over 50%, but keep in mind that its concentration is highly sensitive to temperature. A 50% NaOH solution has about 680 g of NaOH in a volume of 1 L. Do a little calculation and divide 680 g by the molecular weight of NaOH, you'll get ~ 18 moles.
What is the reaction between HCl and water?
What is the reaction between HCl and water? HCl + H2O → H3O+ + Cl. The resulting solution is called hydrochloric acid and is a strong acid. The acid dissociation or ionization constant, Ka, is large, which means HCl dissociates or ionizes practically completely in water. Even in the absence of water, hydrogen chloride can ]
How do you find the concentration of HCl in a titration lab?
0:404:09Titration: Determination of HCl Concentration - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd. So we have sodium chloride salt plus water so the equation is balanced so i have a one-to-oneMoreAnd. So we have sodium chloride salt plus water so the equation is balanced so i have a one-to-one acid to base ratio. That's one-to-one acid base ratio.
How do you find the concentration of HCl?
The calculation of the Molarity of HCl is given using an example of Sol A with the following data:Molarity of NaOH used [M-NaOH] = 0.3M.Dilution factor of HCl [Dil] = 20 (5mL in 100mL))Volume of Acid used in Titration [V-HCl] = 20mL.Volume of NaOH required to neutralise HCl [V-NaOH] = 37.7mL (average of 3 runs)
How do you calculate concentration from a titration?
2:165:13How To Do Titration Calculations | Chemistry | FuseSchool - YouTubeYouTubeStart of suggested clipEnd of suggested clipSolution equals the concentration of the known solution multiplied by the volume of the knownMoreSolution equals the concentration of the known solution multiplied by the volume of the known solution divided by the volume of the unknown.
How do you find the molarity of HCl given molarity of NaOH?
At the equivalence point of the titration, moles NaOH added = moles HCl present, so M(NaOH)*V(NaOH)=M(HCl)*V(HCl).
What concentration is 37% HCl?
12 moles/L12M (37% HCL) = 12 moles/L = 12 x 36.5 = 438 g/L = 438 mg/ml.
How do I calculate the concentration of a solution?
Divide the mass of the solute by the total volume of the solution. Write out the equation C = m/V, where m is the mass of the solute and V is the total volume of the solution. Plug in the values you found for the mass and volume, and divide them to find the concentration of your solution.
How do you find the concentration of acid at the equivalence point?
At the equivalence point the moles of added base will be equal to the moles of original acid, this allows the determination of the number of moles of original acid. This can then be combined with the original volume of the analyte solution to determine its concentration.
What is the concentration of the HCl analyte solution?
Every mole of HCl will produce one mole of H+; therefore, the number of moles of HCl = number of moles of H+. The concentration of the HCl is 0.25 M.
How do you find the concentration of an unknown HCl solution?
1:198:25Chem 60 Experiment 10 Part 2: Molarity of Unknown HCl SolutionYouTubeStart of suggested clipEnd of suggested clipSolution. So once we have our hydrochloric acid in the Erlenmeyer flask. We're going to add ourMoreSolution. So once we have our hydrochloric acid in the Erlenmeyer flask. We're going to add our phenolphthalein indicator. And then titrate with sodium hydroxide.
How do you find the moles of HCl from moles of NaOH?
In the reaction between NaOH and HCl, 1 mole of NaOH reacts with 1 mole of HCl. 1 NaOH + 1 HCl → 1 NaCl + 1 H2O (Mole ratio of NaOH to HCl is 1:1) • The concentration of the NaOH was 0.1 M, or 0.1 moles/liter.
How do you find the concentration of an acid from a titration curve?
Divide the number of moles of analyte present by the original volume of the analyte. For example, if the original volume of the analyte was 500 mL, divide by 1000 mL per L to obtain 0.5 L. Divide 0.01 moles of analyte by 0.5 L to obtain 0.02 moles per liter. This is the concentration or molarity.
How we will determine the concentration of HCl in an unknown solution?
To calculate the concentration of the HCl solution, we just divide the number of moles of HCl by the volume.
How do you find the concentration of an acid?
1:392:30Finding concentration of acid or base given amount needed to titrate and ...YouTubeStart of suggested clipEnd of suggested clipSo to find out M a the molarity of the acid. What the concentration of it what we're going to do isMoreSo to find out M a the molarity of the acid. What the concentration of it what we're going to do is M B VB divided by VA. And that equals.
How do I calculate the concentration of a solution?
Divide the mass of the solute by the total volume of the solution. Write out the equation C = m/V, where m is the mass of the solute and V is the total volume of the solution. Plug in the values you found for the mass and volume, and divide them to find the concentration of your solution.
How do we calculate concentration?
2:004:24Concentration Formula & Calculations | Chemistry | Fuse School - YouTubeYouTubeStart of suggested clipEnd of suggested clipTogether if you want to find the concentration. You cover this and divide the number of moles by theMoreTogether if you want to find the concentration. You cover this and divide the number of moles by the volume. Let's try this. Now. If you have two moles of salt. And dissolve it in two liters of water
How much hydrochloric acid is in 50 ml of titration?
Depending on the titrant concentration (0.2 M or 0.1 M), and assuming 50 mL burette, aliquot taken for titration should contain about 0.26-0.33 g (0.13-0.16 g) of hydrochloric acid (7-9 or 3.5-4.5 millimoles).
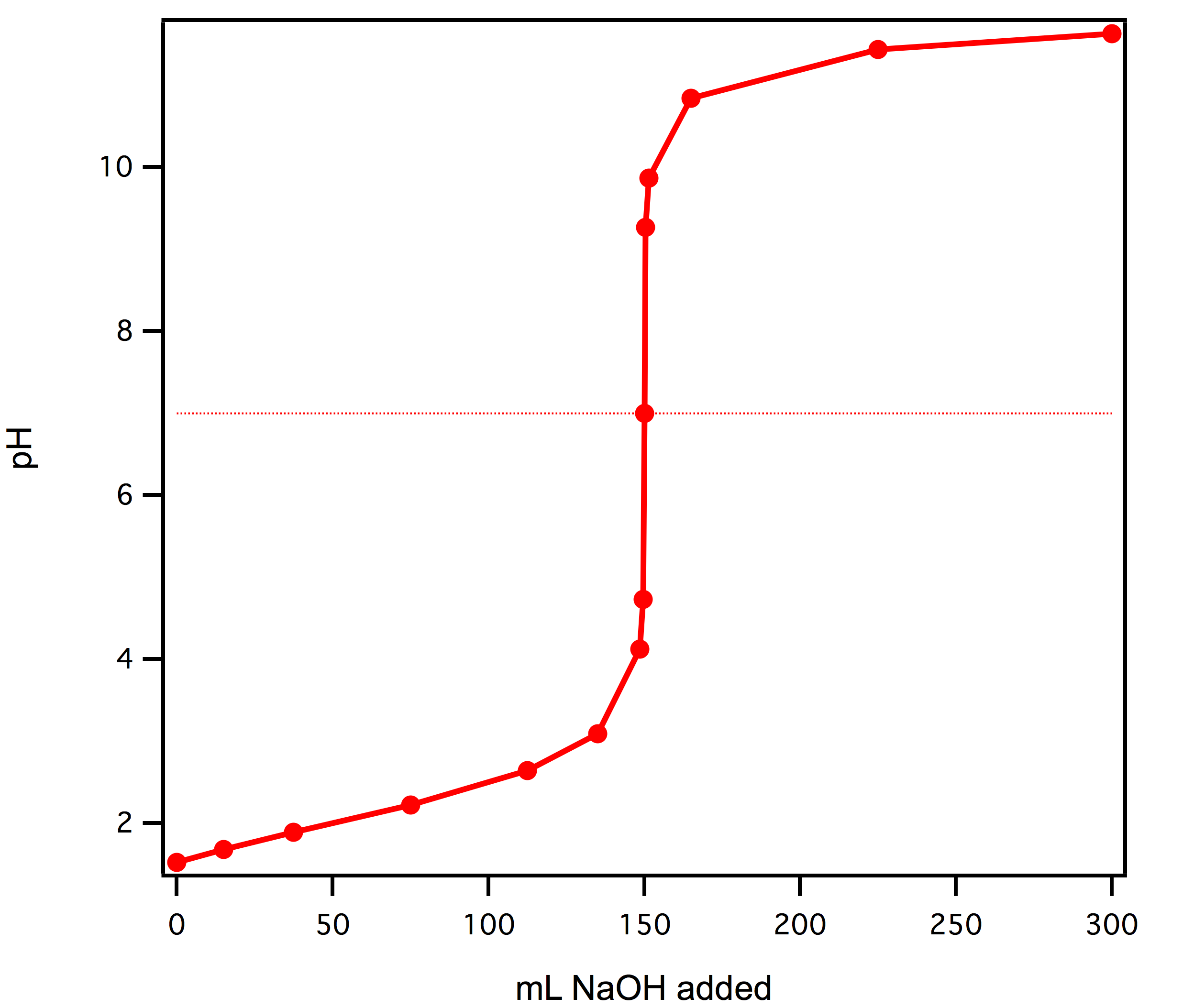
What is the equivalence point of a strong acid titration?
Equivalence point of strong acid titration is usually listed as exactly 7.00. That's not necesarilly the case, as it depends on the solution temperature and ionic strength of the solution, besides, slight hydrolysis of NaOH shifts pH down by about 0.02 unit. Not that it changes much - we are still very close to 7. Thus the best indicator of those listed on pH indicators preparation page is bromothymol blue. However, as we have discussed on the acid-base titration end point detection page, unless we are dealing with a diluted solution (in the range of 0.001 M) we can use almost any indicator that gives observable color change in the pH 4-10 range. Thus we can safely use the most popular phenolphthalein and titrate to the first visible color change.
What is the titrant solution for a titration?
To perform titration we will need titrant - 0.2 M or 0.1 M sodium hydroxide solution, indicator - phenolphthalein solution and some amount of distilled water to dilute hydrochloric acid sample.
What is the dissociation constant of NaOH?
In the reality every acid and every base - no matter how strong - have some dissociation equilibria described by dissociation constant. In this particular case K a for HCl is listed as 10 4 (which means it can be safely neglected) and dissociation constant K b for NaOH is listed as 0.6 - which means sometimes it has to be taken into account.
Is titration of hydrochloric acid strong?
Determination of hydrochloric acid concentration is probably the most often discussed example of acid-base titration. Both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of titration curve and equivalence point are pretty straightforward.
Does hydrochloric acid react with sodium hydroxide?
Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. That makes calculation especially easy - when we calculate number of moles of NaOH used it will be already number of moles of HCl titrated.
Is sodium hydroxide stable?
Sodium hydroxide solutions are not stable as they tend to absorb atmospheric carbon dioxide. Hydrochloric acid is much stronger than carbonic acid, so it will slowly expel carbon dioxide from the solution, but initially presence of carbonates will mean that to reach end point we need to add axcess of titrant.
How much NaOH is needed to titrate 50.00 ml of HCl?
If you know that titrating 50.00 ml of an HCl solution requires 25.00 ml of 1.00 M NaOH, you can calculate the concentration of hydrochloric acid, HCl. Based on the molar ratio between HCl and NaOH, you know that at the equivalence point :
What is acid base titration?
An acid-base titration is a neutralization reaction performed in the lab to determine an unknown concentration of acid or base. The moles of acid will equal the moles of the base at the equivalence point. So if you know one value, you automatically know the other. Here's how to perform the calculation to find your unknown:

General Remarks
Reaction
Sample Size
End Point Detection
Solutions Used
Procedure
Result Calculation
- According to the reaction equation HCl + NaOH → NaCl + H2O Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. That makes calculation especially easy - when we calculate number of moles of NaOH used it will be already number of moles of HCl titrated.
Sources of Errors