
Solution:
- Write the equation for calculating molality: molality = moles (solute) ÷ mass (solvent in kg)
- Rearrange equation to find moles (solute):
- Identify the solute and solvent that make up the solution: solute = sodium chloride = NaCl.
- Calculate moles of solute : moles (solute) = molality × mass (solvent in kg)
How do you calculate molar mass from molality?
Since molality is the number of moles of solute divided by the mass of the solvent in kg, you can calculate the moles of the solute: Divide your mass of solute by the moles of solute and you will get the molar mass (and not 2.989).
How to determine the molar mass of an unknown solute?
We can use a measurement of any one of the following properties to determine the molar mass (molecular weight) of an unknown that is the solute in a solution: Boiling Point Elevation Freezing Point Depression Osmotic Pressure From Boiling Point Elevation
What is molarity of a solution?
Molarity: Molarity is defined as the moles of a solute per liters of a solution. It is used to represent the concentration of the solution represented by M. The following two examples will show how to to find molarity from the solute mass.
How do you calculate the molar mass of sodium sulfide?
Step 1: Calculate the molar mass of the solute from it's chemical formula. Sodium sulfide has two sodium atoms and one sulfur atom. We will take the atomic mass of sodium (22.99 g/mol) multiplied by 2 and add that to the atomic mass of sulfur (32.07 g/mol).
What is the formula for molar mass?
Use Molar Mass Formula. Solution: The molar mass of molecules of those elements is equal to the molar mass of the atoms multiplied by the number of atoms in each molecule. Molar Mass (Cl2) = 2 × 35.453(2) × 1.000000 g/mol = 70.906(4) g/mol.
How do you find the moles of an unknown solute?
0:384:40Determining Molar Mass of Unknown using Freezing Point Depression ...YouTubeStart of suggested clipEnd of suggested clipFor freezing point depression which is the change in freezing point or delta t sub f is going to beMoreFor freezing point depression which is the change in freezing point or delta t sub f is going to be equal to the freezing constant times the molality of the solution.
Why do we need to calculate the molar mass of solutions?
Molar mass is of great importance when setting up an experiment. If you are testing principles involving specific amounts of a substance, the molar mass allows you to figure out how much you should weigh out on your scale.
How do you find moles of solute from molarity?
How do I find moles from molarity?Find the molarity and volume of your solution.Make sure that the units for the volume are the same as for the volume part of the molarity (e.g., mL and mol/mL).Multiply the volume by the molarity. This is the number of moles present.
How do you find the moles of a solution?
First you must calculate the number of moles in this solution, by rearranging the equation. No. Moles (mol) = Molarity (M) x Volume (L) = 0.5 x 2. = 1 mol.For NaCl, the molar mass is 58.44 g/mol. Now we can use the rearranged equation. Mass (g) = No. Moles (mol) x Molar Mass (g/mol) = 1 x 58.44. = 58.44 g.
How do you calculate the number of moles?
The unit is denoted by mol.The formula for the number of moles formula is expressed as.Given.Number of moles formula is.Number of moles = Mass of substance / Mass of one mole.Number of moles = 95 / 86.94.
How do you find the moles of a solvent?
Calculate the moles of the solute by the formula: moles of solute = mass of the solute / molecular mass of the solute compound. Calculate the moles of the solvent by the formula: moles of solvent = mass of the solvent / molecular mass of the solvent compound.
How do you find moles of solute from volume?
There are two steps: Multiply the volume by the density to get the mass. Divide the mass by the molar mass to get the number of moles.
How to Calculate Molarity using Solute Mass
Step 1: Calculate the molar mass of the solute from it's chemical formula.
How to Calculate Molarity using Solute Mass Formulas
Molarity: A measure of concentration with units of moles of solute per liter of solution.
How to Calculate Molarity using Solute Mass Example
If 8.71 g of sodium sulfide ( {eq}Na_ {2}S {/eq}) are dissolved to make 500. mL of solution, what is the molarity of this solution?
How to Calculate Molarity using Solute Mass Example
If 0.51 g of potassium iodide (KI) are dissolved to make 25 mL of solution, what is the molarity of this solution?
How to find the molar mass of a solute?
Divide your mass of solute by the moles of solute and you will get the molar mass (and not 2.989 g).
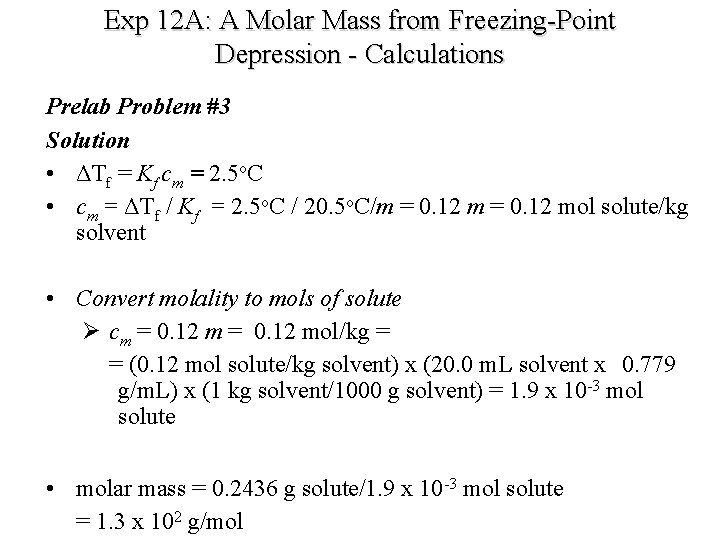
How to find freezing point depression?
The freezing point depression is based on the molal concentration (moles of solute per kg of solvent). You know the freezing point depression of the solution and the cryoscopic constant, so you can calculate the molality: Since molality is the number of moles of solute divided by the mass of the solvent in kg, you can calculate the moles ...
What is the unit of measure of molar mass?
The unit used to measure is grams per mole.
What is the molar mass of hydrogen?
The molar mass of hydrogen is 1.00794 g/mol. The molar mass of oxygen is 15.9994 g/mol. Given that the formula for water is H 2 O, the molar mass would be 2 ( 1.00794) + 15.9994 or 18.01528 g/mol.
What is the mass of sodium hydroxide?
Sodium hydroxide is NaOH.its molar mass involves:mass of Na is-22.9899g/mole,mass of oxygen is 15.999g/mole and mass of hydrogen is 1.0079g/mole you add all these you get 39.996g/.
How much sugar should I put in a cup of tea?
Do you know how sweet it is? No, you need to know how many spoons of sugar were added to know how sweet. I would make 1 cup of tea with 1/2 tsp. sugar. My daughter might put 2 tsp. in her cup, my niece might put 4 tsp. All would differ in sugar concentration and you couldn't calculate a number fo
Do you need to know the concentration of a solute?
No. You need two pieces of info to get concentration, because it is amount of solute per unit volume of solution, or per mass of solution, or mass of solvent. So just the identity of the solute which gives it's molar mass is not enough. You need to know how much of it.
Is water a solvent or a solution?
You can see that water is neither a solution nor a solvent. However some school or maybe if dumb enough, collage teachers might want you to write this answer-
