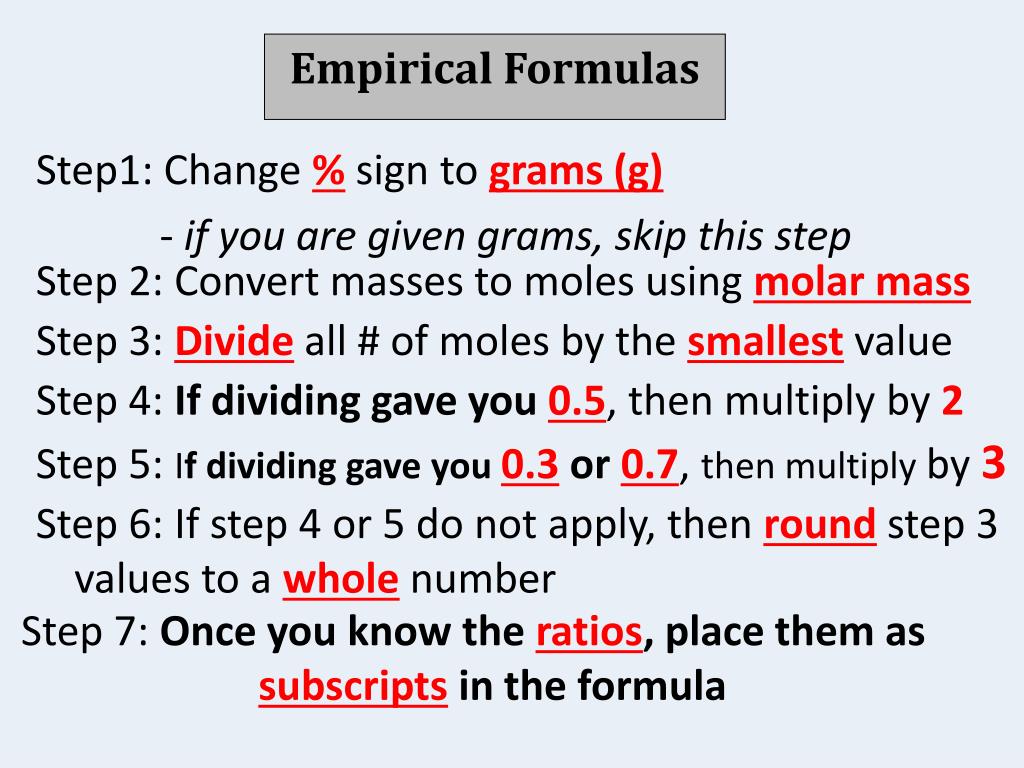
Steps for Finding The Empirical Formula Given Mass Percent
- Change % of each element into grams (for example, if the compound contains 40% carbon, then change it to 40 g carbon)
- Convert grams of each element into moles by dividing grams by molar mass
- Divide all moles by the smallest number of moles
- If the moles are all whole numbers, then you’re done and that’s your empirical formula
How would you find the molecular formula?
Calculate the molar mass based on the formula and divide this into the mass of the actual compound. The division gives you a whole number. Multiply the subscript of each element in the empirical formula by this number to get the molecular formula for the compound.
How do you calculate molecular mass?
Method 1 Method 1 of 2: Calculating Molecular Weight
- Count how many atoms of each element exist in the molecule. First, list each element present in the molecule.
- Find the relative atomic mass of each element in the molecule. Use a copy of the Periodic Table of Elements.
- Calculate the total mass for each element in the molecule. ...
- Add up the mass of all the atoms to find the molecular weight. ...
How can you determine the formula mass of a compound?
To calculate the molar mass of a compound, find the number of grams of each element in one mole of the compound. Then add the masses of the elements in the compound. The decomposition of hydrogen peroxide (H2O2) provides sufficient energy to launch a rocket. What is the molar mass of hydrogen peroxide? 2 mol H × 1g H = 2g H 2 mol O × 16g O = 32g O
How to use empirical formulas to find molecular formulas?
Steps for Finding The Empirical Formula Given Mass Percent
- Change % of each element into grams (for example, if the compound contains 40% carbon, then change it to 40 g carbon)
- Convert grams of each element into moles by dividing grams by molar mass
- Divide all moles by the smallest number of moles
- If the moles are all whole numbers, then you’re done and that’s your empirical formula
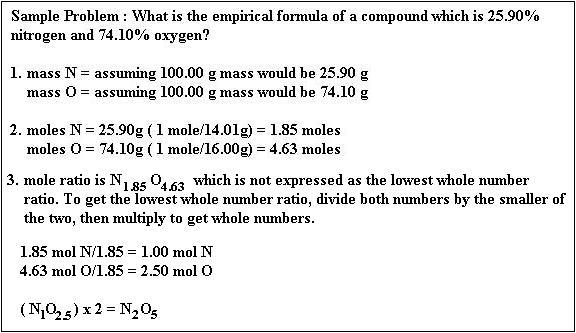
How do you find the molecular formula when given the molar mass?
Divide the molar mass of the compound by the empirical formula molar mass. The result should be a whole number or very close to a whole number. Multiply all the subscripts in the empirical formula by the whole number found in step 2. The result is the molecular formula.
Can you determine molecular formula from percent composition?
Just as the empirical formula of a substance can be used to determine its percent composition, the percent composition of a sample can be used to determine its empirical formula, which can then be used to determine its molecular formula.
How do you find molecular mass from percentages?
For each element, the mass percent formula is:% mass = (mass of element in 1 mole of the compound) / (molar mass of the compound) x 100%mass percent = (mass of solute / mass of solution) x 100%Solution.Answer.Step 1: Find the mass of the individual atoms.More items...•
How do you calculate empirical formula from percentages?
Calculating Empirical Formula from % Composition by MassFirst change all the percentages to grams, and assume 100g of the compound is present. ... Next calculate the number of moles of each element in the compound. ... Then find the empirical formula by dividing the number of moles of each element by the lowest number of moles.
How can the molecular formula be determined?
STEP 1: Calculate the molar mass of the empirical formula. STEP 2: Divide the given molecular molar mass by the molar mass calculated for the empirical formula. STEP 3: Multiply each subscript by the whole number that resulted from step 2. This is now the molecular formula.
How do you find the empirical formula when given mass and percent?
1:155:47Find the Empirical Formula Given Percents - YouTubeYouTubeStart of suggested clipEnd of suggested clipAll you need to do is divide each percent that you're given by the atomic mass of the element.MoreAll you need to do is divide each percent that you're given by the atomic mass of the element. Divide each of those values by whichever one's smallest.
What does the mass percent of an element tell you about a molecule?
Mass percent composition of a molecule shows the amount each element in a molecule contributes to the total molecular mass. Each element's contribution is expressed as a percentage of the whole.
What is the formula of mass by mass percentage of a solution?
Mass PercentMass PercentIn chemistry, the mass fraction of a substance within a mixture is the ratio (alternatively denoted ) of the mass of that substance to the total mass. of the mixture.https://en.wikipedia.org › wiki › Mass_fraction_(chemistry)Mass fraction (chemistry) - Wikipedia: The mass percent is used to express the concentration of a solution when the mass of a solute and the mass of a solution is given: Mass Percent=Mass of SoluteMass of Solution×100%
What is the easiest way to find the empirical formula?
Empirical FormulaBy making use of the molar mass from the periodic table, change the mass of every element to moles.Divide every mole value by the lowest number of moles computed.Round up to the closest whole number. This is denoted by subscripts in the empirical formula and is the mole ratio of the elements.
What are the 3 steps to determining an empirical formula given the percent composition of compound?
Step 1: Determine the masses. Step 2: Determine the number of moles by dividing the grams by the atomic mass. Step 3: Divide the number of moles of each element by the smallest number of moles.
Is percent composition the same as empirical formula?
A compound's percent composition provides the mass percentage of each element in the compound, and it is often experimentally determined and used to derive the compound's empirical formula.
Can you determine the molecular formula from its percent composition quizlet?
Empirical formulas can be determined from the percent composition of a compound. In order to determine its molecular formula, it is necessary to also know the molar mass of the compound.
What does percent composition tell you?
Percent composition is very simple. Percent composition tells you by mass what percent of each element is present in a compound. A chemical compound is the combination of two or more elements.
How are empirical and molecular formulas calculated from percent composition?
1 Answer. take the percentages divide them by the atomic relative mass of the atoms. After dividing you will get the values. Divide all the values with the smallest value which you get and by doing this you will get a ratio and this will be the empirical formula.
Do empirical and molecular formulas of a compound have the same percent compositional values?
Video Transcript. So the answer to the question is, do empirical and molecular formulas of a compound have the same percent compositions. So um the quick answer says yes, they have the same percent compositions.
How to find the molecular formula of a compound?
To be able to find the molecular formula, you’ll need to given the molar mass of the compound. Divide the molar mass of the compound by the molar mass of the empirical formula. This should give you a whole number. Multiply all the subscripts of the empirical formula by the whole number. This will give you the molecular formula.
How to find empirical formula?
Steps for Finding The Empirical Formula Given Mass Percent 1 Change % of each element into grams (for example, if the compound contains 40% carbon, then change it to 40 g carbon) 2 Convert grams of each element into moles by dividing grams by molar mass 3 Divide all moles by the smallest number of moles 4 If the moles are all whole numbers, then you’re done and that’s your empirical formula 5 If not, multiply all moles by number (usually 2 or 3) such that everything is a whole number
How to convert grams into moles?
Convert grams of each element into moles by dividing grams by molar mass. Divide all moles by the smallest number of moles. If the moles are all whole numbers, then you’re done and that’s your empirical formula. If not, multiply all moles by number (usually 2 or 3) such that everything is a whole number.
How to find moles of a compound?
Start by dividing the mass of each element present in the compound by the molar mass of that element to find the number of moles. The periodic table tells you the molar mass of carbon is 12 grams (ignoring fractions), that of hydrogen is 1 gram and that of oxygen is 16 grams. The compound therefore contains 72/12 = 6 moles carbon, 12/1 = 12 moles hydrogen and 96/16 = 6 moles oxygen.
How to find the molecular formula of a compound?
The first step in determining the molecular formula of a compound is to calculate the empirical mass from its empirical formula. To do this, look up the mass of each element present in the compound, and then multiply that number by the subscript that appears after its symbol in the formula. Sum the masses to determine the molar mass represented by ...
How many moles of hydrogen are in formaldehyde?
There are 12 moles of hydrogen but only 6 moles of carbon and oxygen, so divide by 6. The ratios of carbon to hydrogen to oxygen are 1 : 2 : 1, so the empirical formula is CH 2 O, which happens to be the chemical formula for formaldehyde. 2.
What is empirical formula?
The empirical formula for a chemical compound is an expression of the relative abundances of the elements that form it. It isn't the same as the molecular formula, which tells you the actual number of atoms of each element present in a molecule of the compound. Different compounds with very different properties may have the same empirical formula.
How do chemists determine the relative percentages of a compound?
Chemists can determine the elements in a compound and their relative percentages by a chemical reaction with a known compound that produces products that they can collect and weigh. After doing so, they divide the mass of each element by its molar mass to determine the number of moles present in a particular amount – usually 100 grams. ...
What degree does Chris Deziel have?
Chris Deziel holds a Bachelor's degree in physics and a Master's degree in Humanities, He has taught science, math and English at the university level, both in his native Canada and in Japan. He began writing online in 2010, offering information in scientific, cultural and practical topics.
Can you derive the molecular formula of a compound from its empirical formula?
Different compounds with very different properties may have the same empirical formula. You can derive the molecular formula of a compound from its empirical formula only if you know the molar mass of the compound.
Did this article help you?
Cookies make wikiHow better. By continuing to use our site, you agree to our cookie policy.
How to find mole ratio?
Multiply the masses by the mole ratio. Identify how many moles (mole ratio) of each element are in your chemical compounds. The mole ratio is given by the subscript number in the compound. Multiply the molecular mass of each element by this mole ratio.
How to find percentage of vinegar?
You can use the same formula to calculate percentage by volume as you use to calculate percentage by mass. First, calculate the total volume of the solution. Then, divide the volume of the vinegar by the total volume of the solution. Finally, multiply by 100 to get the percentage of vinegar in the total solution. 50 ml + 300 ml = 350 ml for the total volume of the solution. Vinegar percentage = 50/350 = 14 x 100 = 14% vinegar.
How to calculate mass percentage?
In order to calculate the mass percentage, you must know how much salt was added to a certain amount of water. For instance, if you added 50 grams of NaCl to 1000 grams of water, that mass percent would be 50/1050 x 100 = 4.76%.
How does wikihow mark an article as reader approved?
wikiHow marks an article as reader-approved once it receives enough positive feedback. In this case, several readers have written to tell us that this article was helpful to them, earning it our reader-approved status.
How to find the molecular mass of oxygen?
Example 1: Hydrogen has a subscript of two while oxygen has a subscript of 1. Therefore, multiply the molecular mass of Hydrogen by 2, 1.00794 X 2 = 2.01588; and leave the molecular mass of Oxygen as is, 15.9994 (multiplied by one).
How to find the mass percent of a compound?
The basic formula for mass percent of a compound is: mass percent = (mass of chemical/total mass of compound) x 100. You must multiply by 100 at the end to express the value as a percentage.