The percent composition gives you only the empirical formula. Explanation: To get the molecular formula, you must either know the molecular mass or do an experiment to find it.
How do you calculate a molecular formula?
- Start with the number of grams of each element, given in the problem.
- Convert the mass of each element to moles using the molar mass from the periodic table.
- Divide each mole value by the smallest number of moles calculated.
- Round to the nearest whole number. This is the mole ratio of the elements and is.
What are the steps to determine molecular formula?
Terms in this set (35)
- Determine the masses of carbon and hydrogen from the masses of CO2 and H2O respectively.
- Determine the mass of oxygen, if present, by subtracting the masses of carbon adn hydrogen from the total mass
- Determine the number of moles of each element in the compound and hence the empirical formula
How to calculate a molecular formula?
Formula to calculate molecular formula. Divide the molar mass of the compound by the empirical formula molar mass. Multiply all the subscripts in the empirical formula by the whole number found in step 2 ; Example: Lets consider water which has a molar mass of 18g/mol and its empirical formula molar mass is H 2 O.
How would you find the molecular formula?
Calculate the molar mass based on the formula and divide this into the mass of the actual compound. The division gives you a whole number. Multiply the subscript of each element in the empirical formula by this number to get the molecular formula for the compound.
How do you calculate molecular formula?
Divide the molar mass of the compound by the empirical formula molar mass. The result should be a whole number or very close to a whole number. Multiply all the subscripts in the empirical formula by the whole number found in step 2. The result is the molecular formula.
How do you find the empirical formula with percentages?
1:155:47Find the Empirical Formula Given Percents - YouTubeYouTubeStart of suggested clipEnd of suggested clipAll you need to do is divide each percent that you're given by the atomic mass of the element.MoreAll you need to do is divide each percent that you're given by the atomic mass of the element. Divide each of those values by whichever one's smallest.
Can you determine the molecular formula from its percent Composition quizlet?
The percent composition of a compound can also be determined from its chemical formula. The subscripts in the formula are first used to calculate the mass of each element found in one mole of the compound. That value is then divided by the molar mass of the compound and multiplied by 100%.
How do you find the empirical formula from molar mass and percent?
Find the empirical formula.Get the mass of each element by assuming a certain overall mass for the sample (100 g is a good mass to assume when working with percentages). ... Convert the mass of each element to moles. ... Find the ratio of the moles of each element. ... Use the mole ratio to write the empirical fomula.
Are empirical and molecular formulas the same?
There are three main types of chemical formulas: empirical, molecular and structural. Empirical formulas show the simplest whole-number ratio of atoms in a compound, molecular formulas show the number of each type of atom in a molecule, and structural formulas show how the atoms in a molecule are bonded to each other.
How do you convert percentage to Grams?
Converting Percentages Into Grams Divide the percentage by 100, or equivalently, move the decimal place two spots to the left to do this. This means 25 percent is 0.25, 44 percent is 0.44 and 10 percent is 0.1. Using this same method, 8 percent is 0.08.
Can you determine the molecular formula of a substance from its percent composition Why?
Just as the empirical formula of a substance can be used to determine its percent composition, the percent composition of a sample can be used to determine its empirical formula, which can then be used to determine its molecular formula.
Do you always know the molecular formula solely from the percent composition?
Explanation: To get the molecular formula, you must either know the molecular mass or do an experiment to find it. An unknown compound contains 85.63 % C and 14.37 % H .
Can the molecular formula of a compound be determined from its percent composition without additional information?
Yes, you can absolutely determine the molecular formula for its percent composition provided you have the mass of the molecule. To demonstrate, we'll use an easy molecule of water. Water has a molar mass of 18.015 g/mol and a percent composition of 88.81 percent oxygen and 11.19 percent hydrogen.
How do you find molar mass from percent mass?
mass percent = (mass of solute / mass of solution) x 100% The units of mass are typically grams. Mass percent is also known as percent by weight or w/w%. The molar mass is the sum of the masses of all the atoms in one mole of the compound. The sum of all the mass percentages should add up to 100%.
How do you find the number of moles from mass percent?
If you know the percent by weight of a solution, you can find the weight of solute. Divide this by its molecular weight to find the number of moles and divide by the volume of solution to find the molarity.
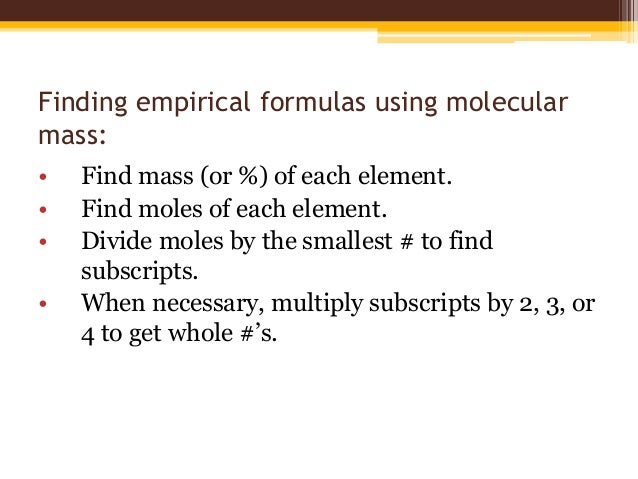
How do you calculate the empirical formula?
Calculate the empirical formula. In any empirical formula problem you must first find the mass % of the elements in the compound. ... Then change the % to grams. ... Next, divide all the masses by their respective molar masses. ... Pick the smallest answer of moles and divide all figures by that.More items...
How is empirical formula determined?
In summary, empirical formulas are derived from experimentally measured element masses by: Deriving the number of moles of each element from its mass. Dividing each element's molar amount by the smallest molar amount to yield subscripts for a tentative empirical formula.
Can the empirical formula of a compound be derived mathematically from Percent Composition data?
You can find the empirical formula of a compound using percent composition data. If you know the total molar mass of the compound, the molecular formula usually can be determined as well.
What c6h12o6 4?
Glucose. Dextrose (D-Glucose) L-Glucose.
How to find subscripts in a formula?
main principle is that the subscripts in the formula are found by calculating mols, not grams or other weight units. So remember, convert to mols by dividing grams of the element by its atomic mass.
How to find the mols of each element?
Very easy.just take each element given,say Carbon (atomic weight =12.0approx.) and divide weight given ,usually in grams to give you mols http://mole.do this for each element given, let’s say next is hydrogen (atomic mass approx = 1.00) this gives you mols of H. Do for each element if more of them and you will have the correct ratios. here’s an example:
What is the molecular mass of ascorbic acid?
The analysis of ascorbic acid (Vitamin C) shows that it contains 40.92% C, 2.58 % and 54.50% O. It’s the molecular mass is 176 g/mole. What is its molecular formula?
What are the elements in a sample?
The sample will LIKELY contain carbon, hydrogen, and nitrogen, and oxygen atoms (and it could contain ONLY these atoms, but a priori we DON’T know), and a known mass of the sample is combusted in a furnace, and the carbon dioxide, water, a...
What is the cyclic structure of NH2?
But it is is a 3D hexagonal cyclic structure (cage like structure similar to adamantane*, C10H16), so to maintain valencies, two N-H bonds of -NH2 are replaced by N—C bonds.
Is molar mass a multiple of formula mass?
Now, there is a real problem. The molar mass MUST be a multiple of the formula mass, which you can see IS NOT the case here.
Can you approximate molar mass?
With only the molar mass I suppose you could , by a lengthy process of elimination , arrive at some formula that approximates the molar mass - but that approach can hardly be called science.
How to find the molecular formula of a compound?
To be able to find the molecular formula, you’ll need to given the molar mass of the compound. Divide the molar mass of the compound by the molar mass of the empirical formula. This should give you a whole number. Multiply all the subscripts of the empirical formula by the whole number. This will give you the molecular formula.
How to find empirical formula?
Steps for Finding The Empirical Formula Given Mass Percent 1 Change % of each element into grams (for example, if the compound contains 40% carbon, then change it to 40 g carbon) 2 Convert grams of each element into moles by dividing grams by molar mass 3 Divide all moles by the smallest number of moles 4 If the moles are all whole numbers, then you’re done and that’s your empirical formula 5 If not, multiply all moles by number (usually 2 or 3) such that everything is a whole number
How to convert grams into moles?
Convert grams of each element into moles by dividing grams by molar mass. Divide all moles by the smallest number of moles. If the moles are all whole numbers, then you’re done and that’s your empirical formula. If not, multiply all moles by number (usually 2 or 3) such that everything is a whole number.
What is the formula for cassiterite?
The ratios indicate that there is one tin atom for every two oxygen atoms. Thus, the simplest formula of cassiterite is SnO2.
How to find the whole number ratio of a vitamin C?
To find the simplest whole number ratio, divide each number by the smallest number of moles: C: 3.41 / 3.41 = 1.00. H: 4.53 / 3.41 = 1.33.
What are the elements in vitamin C?
Vitamin C contains three elements: carbon, hydrogen, and oxygen. Analysis of pure vitamin C indicates that the elements are present in the following mass percentages: C = 40.9. H = 4.58. O = 54.5. Use the data to determine the simplest formula for vitamin C.
What is the mass percentage of cassiterite?
Chemical analysis of cassiterite shows that the mass percentages of tin and oxygen are 78.8 and 21.2, respectively . Determine the formula of this compound.
How many hydrogen atoms are in 1.33?
Also, there are 1.33 = 4/3 hydrogen atoms. (Note: converting the decimal to a fraction is a matter of practice! You know the elements must be present in whole number ratios, so look for common fractions and become familiar with the decimal equivalents for fractions so you can recognize them.)
What is the molar ratio of cadium acetate?
Notice how the molar ratio in the full formula for cadium acetate is 1 : 4 : 6 : 4
Is empirical formula the same as molecular formula?
the empirical formula is also the molecular formula
How to find the molecular formula of a compound?
We can use the empirical formula to find the molecular formula using the molecular weight of the compound and the molecular weight of the empirical formula.
What is the difference between empirical and molecular formulas?
The empirical formula gives the smallest whole number ratio between elements in a compound. The molecular formula gives the actual whole number ratio between elements in a compound. For some molecules, the empirical and molecular formulas are the same. Usually, the molecular formula is a multiple of the empirical formula.
What is empirical formula?
The empirical formula tells us the ratio between atoms of the elements, which can indicate the type of molecule (a carbohydrate, in the example). The molecular formula lists the numbers of each type of element and can be used in writing and balancing chemical equations. However, neither formula indicates the arrangement of atoms in a molecule.
How many moles of oxygen are there in a mole of hydrogen?
We have all the information we need to write the empirical formula. For every two moles of hydrogen, there is one mole of carbon and one mole of oxygen.
How many empirical formula units does it take to make a compound?
It takes six empirical formula units to make the compound, so multiply each number in the empirical formula by 6.
Why is 100 grams used for a sample size?
Note: 100 grams is used for a sample size just to make the math easier. Any sample size could be used, the ratios between the elements will remain the same.
How to find the mass of an element in one mole of a compound?
Mass of an element in one mole of the compound = ( Its percentage × Molar mass of the compound ) ÷ 100%
What is the chemical formula of a compound?
The chemical compound is represented by a chemical formula which is a simple symbolic formula that indicates the element percentage and the number of atoms or ions of each element , The chemical compound is made up of units called molecules or formula units which consist of atoms or ions of two or more elements.
What is the empirical formula for P4O10?
Applications : P2O5 is the empirical formula for P4O10 , CH2O is the empirical formula for CH2O , C2H4O2 , C6H12O6
Why does empirical formula not represent the chemical structure of a compound?
In most cases the empirical formula can not represent the chemical structure of a compound because the empirical formula does not represent the actual number of atoms , ions or formula units which form the molecule of the compound . Both of actylene C2H2 and benzene C6H6 have the same empirical formula CH , but they differ in ...
Why is the empirical formula for carbon monoxide the same as the molecular formula for nitric oxide?
The molecular formula and the empirical formula for each of carbon monoxide CO and nitric oxide NO are the same because the molar mass of the empirical formula equal the molar mass of the molecular formula . In most cases the empirical formula can not represent the chemical structure of a compound because the empirical formula does not represent ...
What are the different types of chemical formulas?
Types of chemical formulas. The types of chemical formulas are the empirical formula , The molecular formula and the structural formula .
Which compounds have the same empirical formula?
In some cases many compounds may have the same empirical formula such as : Acetylene C2H2 and benzene C6H6 have the same empirical formula ( CH ) .