
The quotient gives the value of the number of sigma bond pairs and the remainder divided by 2 gives the number of lone pairs. The number of valance electrons counted divided by 8 will give the number of sigma bonds formed.
How to determine the number of lone pairs of an atom?
To summarize, when asked to determine the number of lone pairs, remember that electrons are associated with a negative charge so if the atom is negatively charged, consider the possibility of having alone pair (s). Likewise, a partial positive charge should be a hint that the atom does not have as many bonds (electrons) as it should.
What is the easiest way to find Bond pair and lone pair?
- Quora What is the easiest way to find bond pair and lone pair in chemical bonding? By knowing the structure of the compound you can easily identify the bond pair and lone pairs in a compound.
How do you find the number of Siga bonds in a compound?
There is no such way (unless you look at chemistry with an intellectual approach) For me , the easiest way is to identify the central atom in the compound and hence find the number of siga bonds it forms. After that look for the lone pairs.
How do you calculate lone pairs of oxygen in H2O?
Again in H2O there are 2 H atoms attached to oxygen so there are 4 electrons left which forms two lone pairs. Moreover you can see it this way Lone Pair= (no of valance shell electrons of the central atom - no of electrons taken part in bonding)/2. Hope this helps. There is no such way (unless you look at chemistry with an intellectual approach)
How do you calculate bonding pairs?
Steps for Counting Bonding Electron Pairs in a Lewis StructureStep 1: Count each single line as one bonded pair of electrons.Step 2: Count each double line as two bonded pairs of electrons.Step 3: Count each triple line as three bonded pairs of electrons.Step 4: Add up the numbers from each step.
How many bond pairs and lone pairs each molecule have?
A molecule has 3 bonded pairs and 2 lone pairs on the central atom.
What is lone pair formula?
A negatively charged carbon atom should immediately tell you about a lone pair of electrons. In this case, since the carbon has only three bonds and a negative charge, it must also have a lone pair. This can also be confirmed by using the formula: FC= V – (N + B)
How many bond pairs and lone pairs are in H2O?
AB2E2: Water, H2O A water molecule consists of two bonding pairs and two lone pairs (see figure below).
How do you determine the number of bonding groups?
Solution Draw the Lewis Structure. Count the total number of bonds. The total number of bonds is 4. Count the number of bond groups between individual atoms. The number of bond groups between atoms is 2. Divide the bond groups between individual atoms by the total number of bonds.
What is the easiest way to find lone pairs?
Find the number of lone pairs on the central atom by subtracting the number of valence electrons on bonded atoms (Step 2) from the total number of valence electrons (Step 1). Divide the number of VEs not in bonds (from Step 3) by 2 to find the number of LPs.
How many lone pairs are in nh3?
one lone pairThere are just three bonded groups, therefore there is one lone pair. However since the lone pairs are 'invisible', the shape of ammonia is pyramidal. The geometry of ammonia, NH3.
How many lone pairs are in co2?
two lone pairsHow many lone pairs are there in the Lewis structure CO2? Each oxygen atom in the CO2 molecule has two lone pairs of electrons.
What are bonding pairs and lone pairs?
The difference between bond pair and lone pair is that a bond pair is composed of two electrons that are in a bond whereas a lone pair is composed of two electrons that are not in a bond.
How many lone pairs does NH3 have?
one lone pairThere are just three bonded groups, therefore there is one lone pair. However since the lone pairs are 'invisible', the shape of ammonia is pyramidal. The geometry of ammonia, NH3.
Why does h2o have 2 lone pairs?
The water molecule is so abundant that only memorising that water is a bent molecule is wise. In order to complete its octet, oxygen has 6 valence electrons and therefore requires 2 additional electrons from 2 hydrogen atoms. This also leaves two pairs of lone electrons which are not bound to any other atoms.
How many lone pairs does co2 have?
How many lone pairs are there in the Lewis structure CO2? Each oxygen atom in the CO2 molecule has two lone pairs of electrons.
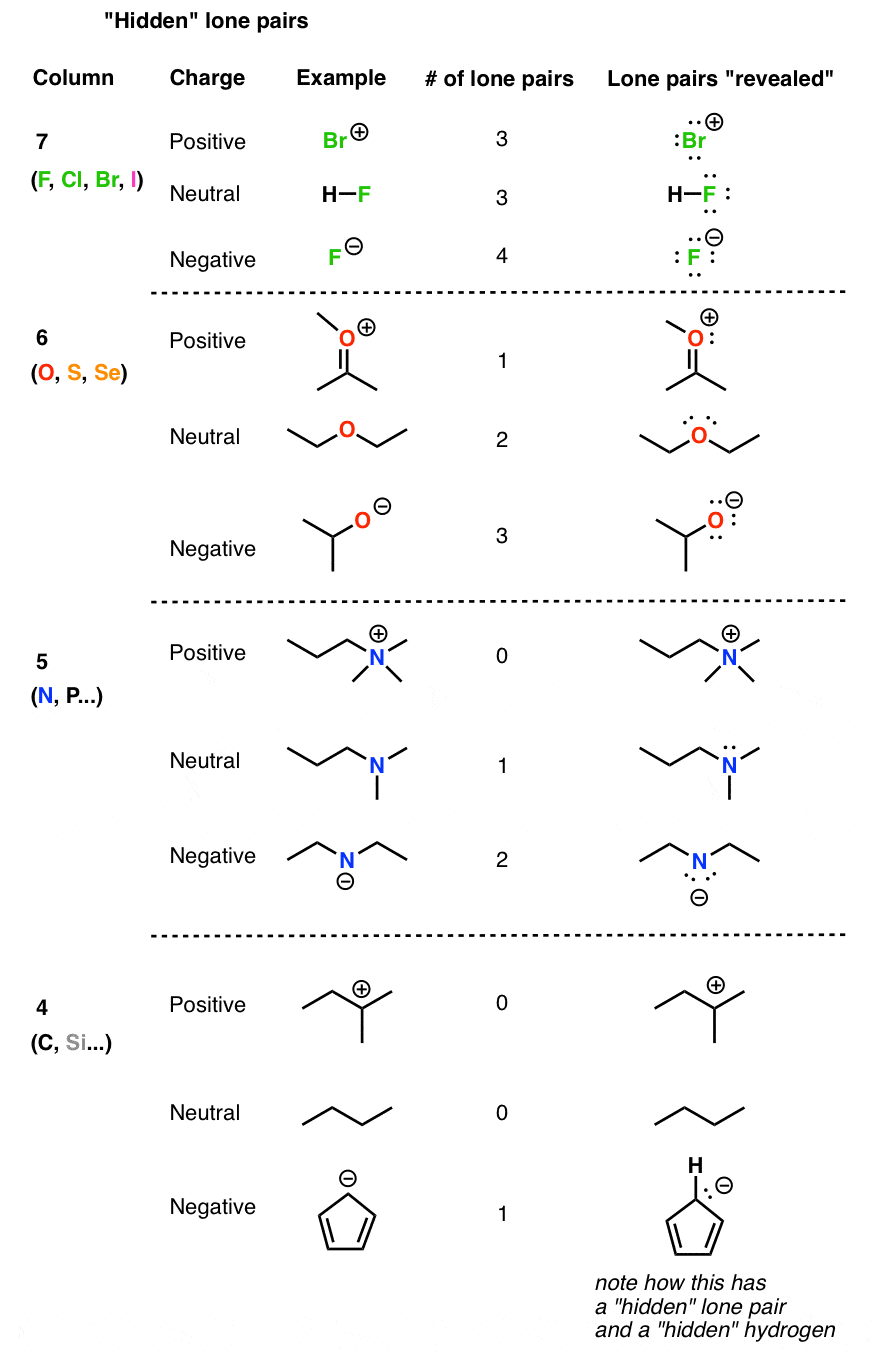
A Formula to Determine The Number of Lone Pairs
Determining The Number of Lone Pairs When There Is A Formal Charge
- Let’s now consider this question when there is one more hydrogen bonded to the oxygen. How many lone pairs does the oxygen have in the following molecule? What we notice first is the positive charge on the oxygen which is going to affect the number of lone pairs. The positive charge indicates that one of the lone pairs that was initially there was used for making the extra …
Number of Lone Pairs with Negative Partial Charge
- Contrary to the positive charge, a negative partial charge accounts for an additional one pair. For example, carbon is normally associated with four bonds and no lone pairs. However, when bonded with metals, the carbon is often said to be negatively charged because of the large difference in the electronegativity valueseven though these bonds are often covalent. Later, in your organic ch…