PV Diagram: Work Done = Area Under or Enclosed by Curve
- The work done by a system can be calculated by the area under a curve in a pressure-volume diagram.
- A reversible process can produce the greatest amount of work.
How do we determine the sign of the work done from a PV diagram?
What do solved examples involving PV diagrams look like?
How do we heat up a gas?
What does each point on a PV diagram represent?
What happens if the path on a PV diagram is directed to the left?
How to visualize gas pressure and volume?
What happens if you see a state shifting to the left on a PV diagram?
See 4 more
About this website
How do you find the work done on a PV diagram?
0:3920:16PV Diagrams, How To Calculate The Work Done By a Gas ... - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd to calculate the work performed by the gas is going to equal the pressure times the change inMoreAnd to calculate the work performed by the gas is going to equal the pressure times the change in volume.
What is work on a PV diagram?
For a cyclic process, PV graph is a closed curve and the area enclosed by the curve is the work done. If the cycle is clockwise work done is positive, and if the cycle is anticlockwise work done is negative.
What is the equation for PV work?
Work done by gases is sometimes called pressure-volume or PV work. To calculate how much work is done (or has done to it) by the gas against a constant external pressure, we have to use an equation: WORK= (w) = -Pexternal×△V where external pressure is denoted by Pexternal.
Is work done equal to PV?
Pressure-volume work: Work done by a gas Gases can do work through expansion or compression against a constant external pressure. Work done by gases is also sometimes called pressure-volume or PV work for reasons that will hopefully become more clear in this section!
Is work done area under PV graph?
We can find the work done by determining the total area under the curve on a PV diagram. We have to make sure we use the total area, all the way down to the volume axis. For instance, we can imagine viewing the area under the curve in the example shown above as a triangle and a rectangle (as seen below).
What is W =- P Delta V?
w=−PΔV. The negative sign associated with PV work done indicates that the system loses energy. If the volume increases at constant pressure (ΔV > 0), the work done by the system is negative, indicating that a system has lost energy by performing work on its surroundings.
How do you find work done by volume?
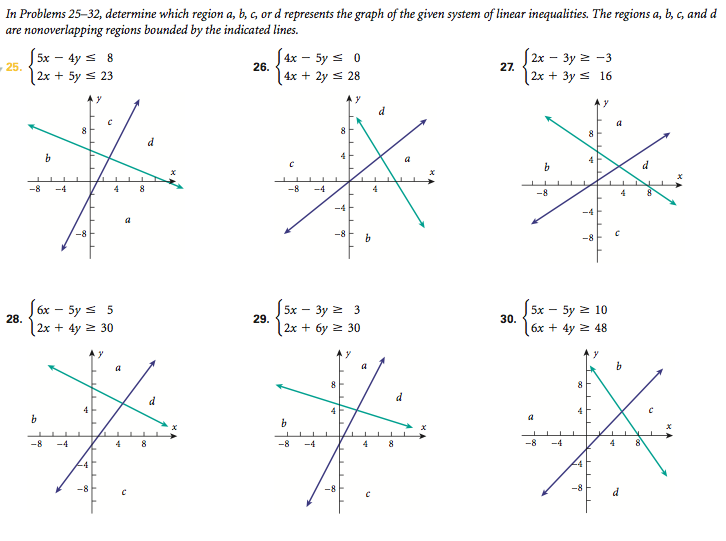
0:054:42Work Done by a Gas During Pressure and Volume Change - YouTubeYouTubeStart of suggested clipEnd of suggested clipDuring a pressure and volume. Change question one reads two moles of an ideal gas maintained at 20MoreDuring a pressure and volume. Change question one reads two moles of an ideal gas maintained at 20 degrees celsius expand until the pressure is one half of the original. How much work is done by the
How does one calculate the work done by the Environment on gas using a PV diagram?
W=PΔV(isobaric process). W = P Δ V (isobaric process). Note that if ΔV is positive, then W is positive, meaning that work is done by the gas on the outside world.
What is work done by a system?
In terms of physics, if force is applied on a system and the system experiences a displacement then the work is said to be done on the system. If a system applies a force on something else and causes displacement then the work is said to be done by the system.
What is work done per charge?
The work per unit of charge is defined by moving a negligible test charge between two points, and is expressed as the difference in electric potential at those points. The work can be done, for example, by electrochemical devices (electrochemical cells) or different metals junctions generating an electromotive force.
Is work done total energy?
Connection Between Energy and Work Done In other words, the work done is equal to the change in energy. For example, the work done against gravity is equal to the change in the potential energy of the body and the work done against all resistive forces is equal to the change in the total energy.
What formula is Q MC ∆ T?
The amount of heat gained or lost by a sample (q) can be calculated using the equation q = mcΔT, where m is the mass of the sample, c is the specific heat, and ΔT is the temperature change.
What is work in thermodynamic system?
In thermodynamics, thermodynamic work is the quantity of energy transferred from one system to another. It is a generalization of the concept of mechanical work in mechanics. In the SI system of measurement, work is measured in joules (symbol: J). The rate at which work is performed is power.
What principle is at work in solar panels?
the photovoltaic effectThey work on the principle of the photovoltaic effect. When certain materials are exposed to light, they absorb photons and release free electrons. This phenomenon is called as the photoelectric effect.
What is the work done in cyclic process as shown in PV diagram?
For cyclic process, the work done is W(net)=4π(P2−P1)(V2−V1)
What is work done in adiabatic system?
The equation for an adiabatic process can be derived from the first law of thermodynamics relating to the change in internal energy dU to the work W done by the system and the heat dQ added to it. The work done dW for the change in volume V by dV is given as PdV.
The pressure-volume (pV) diagram and how work is produced in an ICE
The internal combustion engine is a heat engine.It’s working principle is based on the variation of pressure and volume inside the engine’s cylinders. All heat engines are characterized by a pressure-volume diagram, also known as pV diagram, which basically shows the variation of the pressure in the cylinder function of its volume, for a complete engine cycle.
P–V diagram & calculation of work done - PhysicsTeacher.in
In this post, we will discuss how the P-V diagram can help us to calculate the work done by a system or on a system. We will derive the equation of work done in terms of pressure and volume. We will solve a few numerical problems as well based on P-V diagrams.
PV Diagrams | Temperature, Kinetic Theory, and Gas Laws - Nigerian Scholars
PV Diagrams. We can examine aspects of the behavior of a substance by plotting a graph of pressure versus volume, called a PV diagram.When the substance behaves like an ideal gas, the ideal gas law describes the relationship between its pressure and volume.
How to calculate the temperature in a P-V diagram - Quora
Answer: It depends on what is the equation of state for the physical system you are modeling in the P-V diagram. For example, the equation of state for an ideal gas is PV = nRT. So if your process has a fixed amount of the gas (constant number of moles, n) you can calculate the temperature T for ...
How do we determine the sign of the work done from a PV diagram?
Let's say our gas starts out in the state shown in the PV diagram below.
What do solved examples involving PV diagrams look like?
An ideal gas in a sealed container is taken through the process shown in the PV diagram below.
How do we heat up a gas?
We can do work on the gas by pressing the piston downward, and we can heat up the gas by placing the container over a flame or submerging it in a bath of boiling water. When we subject the gas to these thermodynamics processes, the pressure and volume of the gas can change.
What does each point on a PV diagram represent?
Every point on a PV diagram represents a different state for the gas (one for every possible volume and pressure). As a gas goes through a thermodynamics process, the state of the gas will shift around in the PV diagram, tracing out a path as it moves (as shown in the diagram below).
What happens if the path on a PV diagram is directed to the left?
If the path on a PV diagram is directed to the left, the volume is decreasing, and positive work is being done on the gas. If the path on a PV diagram is directed to the right (as in the diagram above), the volume is increasing, and negative work is being done on the gas since . It doesn't matter what shape the path takes, ...
How to visualize gas pressure and volume?
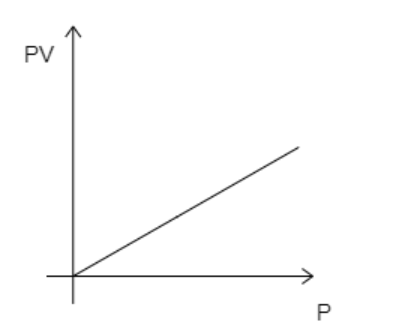
A convenient way to visualize these changes in the pressure and volume is by using a Pressure Volume diagram or PV diagram for short. Each point on a PV diagram corresponds to a different state of the gas. The pressure is given on the vertical axis and the volume is given on the horizontal axis, as seen below.
What happens if you see a state shifting to the left on a PV diagram?
So if we ever see a state shifting to the left on a PV diagram we can say for sure that the work done on the gas was positive. Similarly, if we ever see a state shifting to the right on a PV diagram we can say for sure that the work done on the gas was negative.
What is the area under the curve of a pressure versus volume graph?
The area under the curve equals the work done by the gas, since W =P ΔV W = P Δ V.
What is the process of gas working on a piston at constant pressure called?
A process by which a gas does work on a piston at constant pressure is called an isobaric process. Since the pressure is constant, the force exerted is constant and the work done is given as
What is the work done in isobaric expansion?
An isobaric expansion of a gas requires heat transfer to keep the pressure constant. Since pressure is constant, the work done is P ΔV P Δ V.
How do we determine the sign of the work done from a PV diagram?
Let's say our gas starts out in the state shown in the PV diagram below.
What do solved examples involving PV diagrams look like?
An ideal gas in a sealed container is taken through the process shown in the PV diagram below.
How do we heat up a gas?
We can do work on the gas by pressing the piston downward, and we can heat up the gas by placing the container over a flame or submerging it in a bath of boiling water. When we subject the gas to these thermodynamics processes, the pressure and volume of the gas can change.
What does each point on a PV diagram represent?
Every point on a PV diagram represents a different state for the gas (one for every possible volume and pressure). As a gas goes through a thermodynamics process, the state of the gas will shift around in the PV diagram, tracing out a path as it moves (as shown in the diagram below).
What happens if the path on a PV diagram is directed to the left?
If the path on a PV diagram is directed to the left, the volume is decreasing, and positive work is being done on the gas. If the path on a PV diagram is directed to the right (as in the diagram above), the volume is increasing, and negative work is being done on the gas since . It doesn't matter what shape the path takes, ...
How to visualize gas pressure and volume?
A convenient way to visualize these changes in the pressure and volume is by using a Pressure Volume diagram or PV diagram for short. Each point on a PV diagram corresponds to a different state of the gas. The pressure is given on the vertical axis and the volume is given on the horizontal axis, as seen below.
What happens if you see a state shifting to the left on a PV diagram?
So if we ever see a state shifting to the left on a PV diagram we can say for sure that the work done on the gas was positive. Similarly, if we ever see a state shifting to the right on a PV diagram we can say for sure that the work done on the gas was negative.