
How could you distinguish an element from a compound?
How could you distinguish an element from a compound? If it is on the periodic table, it is an element. An element is a pure substance and is found on the periodic table. If you have more than one element chemically bonded, it is a compound. Hydrogen, oxygen, and iron are all elements.
How is a compound represented in chemistry?
A compound is represented using its chemical formula that represents the symbols of its constituent elements and the number of atoms of each element in one molecule of the compound. An element is represented using symbols. Composition.
How are elements represented in a chemical formula?
A compound is represented using its chemical formula that represents the symbols of its constituent elements and the number of atoms of each element in one molecule of the compound. An element is represented using symbols. Elements are distinguished by their name, symbol, atomic number, melting point, boiling point, density and ionization energies.
What are the characteristics of compounds?
Compounds have fixed melting and boiling points. They do not show the properties of their constituent elements. A compound cannot be broken down into its elements by simple physical methods. Example: Water is a compound. We cannot separate the hydrogen and oxygen present in it by using physical methods.
What are Compounds?
Why do compounds have a molecular formula?
How are covalent compounds formed?
What are the physical properties of non-metals?
What are impure substances?
What is an ionic compound?
Which element has the tendency to lose electrons in order to gain stability?
See 4 more
About this website
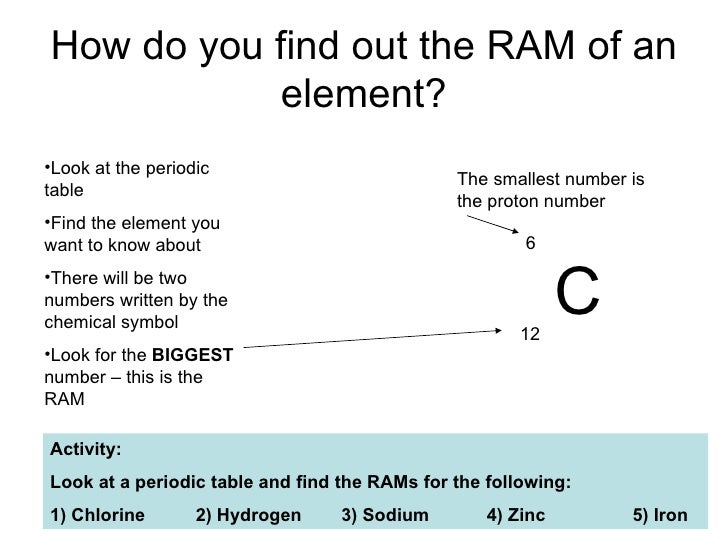
How do you identify an element?
An element is defined by the number of protons in one atom. For example, every single atom of hydrogen has exactly one proton. We say that hydrogen has a proton number or atomic number of 1. The periodic table is arranged in order of proton number, which is why hydrogen is in the very first box with a 1 next to it.
How can we detect and identify elements and compounds?
Mass spectrometry is useful for identifying elements and the relative ratio of isotopes. For molecules, it can help to determine a molecular structure. The atoms or molecules are vaporised and converted to positive ions (based on a single atom or molecular fragment) by bombardment with high energy electrons.
What is the basic step in differentiating an element from a compound?
An element is a substance that cannot be chemically decomposed into simpler substances. An atom is the smallest particle of an element that can take part in chemical reactions. A compound is a substance formed from two or more elements chemically joined (bonded) together.
How do you determine the state of an element or compound easily?
2:434:31HOW TO FIGURE OUT THE STATE OF AN ELEMENT OR COMPOUNDYouTubeStart of suggested clipEnd of suggested clipIf it is soluble it is aqueous. If it is insoluble it is a solid if it is soluble. But part of theMoreIf it is soluble it is aqueous. If it is insoluble it is a solid if it is soluble. But part of the compound is an exception it is a solid if it is insoluble.
How do scientists identify compounds?
Although the instrumentation can become fairly complex, spectroscopy is generally a more reliable method for identifying atoms and molecules. These methods can identify a larger range of chemicals and chemicals present in lower concentrations in comparison to chemical tests alone.
Is it possible to distinguish between an element and a compound?
Yes, because elements is the smallest and simplest form of matter that can't be broken into simpler substance by any physical or chemical means, while compound is a combination of two or more elements that are combined together with chemical bonds.
How are elements identified in terms of their atoms?
How are elements described in terms of their atoms? Each element is made up of atoms that differ from the atoms of other elements. An element can be identified by the number of protons in the nucleus of its atoms.
How could you distinguish an element from a compound quizlet?
How could you distinguish an element from a compound? Compounds can be separated by chemical means into simpler substance and elements can not at all.
Difference Between Element and Compound - BYJUS
Sl. No. Differentiating Property Element Compound; 1: Definition: Elements are pure substances which are composed of only one type of atom. Compound are substances which are formed by two or more different types of elements that are united chemically in fixed proportions.
Elements Compounds and Molecules: Definition,Concepts,Difference
All pure substances are classified as either elements or compounds. In this article, we will study what are elements, compounds and molecules, Laws of Chemical Combinations, Law of Conservation of Mass, Law of Definite Composition, Law of Multiple Proportions, Gay Lussac’s Law of Combining Volumes of Gases, and Avogadro’s Law
ELEMENTS, COMPOUNDS & MIXTURES
3 Li 1 ium ium V s 1 H 8 n step. 2 He n3 m Helium 3 Li 1 ium ium 4 Be 2 ium m Y U Ir 5 Ga B 1 n 6 in C 1 n) 7 N 1 n s 8 O 0 n m 9 Cl F 0 e 10 Ne uo8 n 11 Na 9 m 12 Mg 1 13 Dy Al 8 iumm m 14 Si 9 icon 15 P 7 c 16
What are Compounds?
A compound is a pure substance that is formed by the chemical combination of two or more elements in a fixed proportion by mass. Compound formation always takes place as a result of a chemical reaction. Therefore, a compound does not exhibit the properties of its constituent elements. On breaking down the compound, the smallest entity so obtained is called a molecule of the compound.
Why do compounds have a molecular formula?
Solution: Compounds are pure substances whereas mixtures are impure. A compound always has a fixed chemical composition by mass, which means that the elements that combine together to form a compound always do so in a fixed ratio. This is why compounds have a molecular formula. Compounds have fixed melting and boiling points. They do not show the properties of their constituent elements. A compound cannot be broken down into its elements by simple physical methods.
How are covalent compounds formed?
They are also called molecular compounds. They are formed as a result of the sharing of electrons between two or more non-metals.
What are the physical properties of non-metals?
The physical properties of non-metals include brittleness, comparatively lower tensile strength, non-luster, non-conductivity or insulation, lower melting and boiling points, etc.
What are impure substances?
Pure substances can further be classified into elements and compounds. Impure substances are also called mixtures. Depending on their composition, they can further be classified into homogeneous and heterogeneous.
What is an ionic compound?
Ionic compounds are formed between a metal and a non-metal. They are also called electrovalent compounds as they involve ions. The metal atom loses electrons to form a cation, and the non-metal atom gains the electron to form an anion.
Which element has the tendency to lose electrons in order to gain stability?
Metals are the elements that have the tendency of losing electrons in order to gain stability, that is, they exhibit electropositivity. The physical properties of metals include hardness, high tensile strength, luster, conductivity, high melting and boiling points, etc.
How to find the electron count of an element?
This number is the atomic number of the element, which you can look up on the periodic table.
What is the number next to each element in the periodic table?
Most periodic tables show two numbers next to each element. The smaller one (that always goes up by 1 as you go left to right) is the atomic number or proton number. The larger number is the atomic mass.
What is the atomic number of hydrogen?
We say that hydrogen has a proton number or atomic number of 1. The periodic table is arranged in order of proton number, which is why hydrogen is in the very first box with a 1 next to it. Atomic number is abbreviated "Z".
What does a lithium spectrum look like?
For example, a lithium spectrum has a very bright, thick green line, and several other fainter ones in different colors. If your spectrum has all those same lines on it, the light came from the element lithium. (Some types of spectra will show dark gaps instead of bright lines, but you can compare these the same way.)
What does the smallest bar on a mass spectrometer mean?
The smallest bars often represent small amounts of more charged particles that you can ignore for identification purposes.
Which element has 8 electrons?
For example, if you are asked which element has 8 electrons, look for the element with atomic number 8: oxygen.
Which element fills up the 1s orbital?
The first row (hydrogen and helium) fills up the 1s orbital from left to right. Think of these, plus all elements in the first two columns, as the "s-block". Each row of the "s-block" fills up one s orbital. The right-hand side of the table is the "p-block", starting with boron through neon.
What is the difference between an element and a compound?
The difference between an element and a compound is that an element is a substance made of same type of atoms, whereas a compound is made of different elements in definite proportions. Examples of elements include iron, copper, hydrogen and oxygen.
How is a compound represented?
Representation. A compound is represented using its chemical formula that represents the symbols of its constituent elements and the number of atoms of each element in one molecule of the compound. An element is represented using symbols. Examples.
What is the atomic number of an element?
Atomic number – the atomic number is denoted by the letter Z and is the number of protons present in the nucleus of the atom of element. For e.g. carbon has 6 protons in its nucleus and for Carbon, Z = 6. Number of protons is also indicative of electric charge or number of electrons present in the nucleus which determines chemical properties ...
What is the valency of an element?
Valency is defined as the number of hydrogen atoms required that can combine with an atom of the element forming the compound. Most compounds can exist as solids (low enough temperatures) and can be decomposed by the application of heat.
How are compounds made?
Compounds are composed of different elements in a fixed proportion. For example, 1 atom of sodium (Na) combines with 1 atom of chlorine (Cl) to form one molecule of sodium chloride (NaCl) compound. The elements in a compound do not always retain their original properties and cannot be separated by physical means. The combining of elements is facilitated by their valency. Valency is defined as the number of hydrogen atoms required that can combine with an atom of the element forming the compound. Most compounds can exist as solids (low enough temperatures) and can be decomposed by the application of heat. Sometimes foreign elements are trapped inside crystal structure of compounds giving them a non homogeneous structure. Compounds are depicted by their chemical formula which follows the Hill system wherein carbon atoms are listed first, followed by hydrogen atoms after which elements are listed in alphabetical order.
How many elements are there in the periodic table?
Elements are listed according to their atomic number on the Periodic Table. Among the 117 known elements, 94 are naturally occurring like carbon, oxygen, hydrogen etc. 22 are artificially produced having undergone radioactive changes. The reason for this is their instability due to which they undergo radioactive decay over a period of time giving rise to new elements during the process like Uranium, Thorium, Bismuth etc. Elements combine in fixed ratios and give rise to stable compounds due to chemical bonds that facilitate compound formation.
What is the meaning of compound?
Before the 1800s the usage of term compound could also mean a mixture. It was in the 19th century that meaning of a compound could be distinguished from a mixture . Alchemists like Joseph Louis Proust, Dalton and Berthollet and their studies on various compounds have given modern chemistry the current definition of compound. Proust’s work demonstrated to the world of chemistry that compounds were made constant composition of respective elements.
How to write the formula of a compound?
Step-1 : Write the symbols of formulae of the ions of the compound side by side with positive ion on the left hand side and negative ion on right hand side. Step-2 : Enclose the polyatomic ion in a bracket. Step-3 : Write the valency of each ion below its symbol. Step-4 :
What is the chemical formula of a substance?
A chemical formula is a representation of a chemical substance using letters for atoms and subscript numbers to show the numbers of each type of atoms that are present in the substance. Based on the chemical formula of a substance, we know the composition of the substance. (a) The elements that make up the substance.
How many atoms are in a molecule of hydrogen?
Subscript number at the right bottom shows the number of atoms in each molecule. Example: The formula H 2 indicates that one molecule of hydrogen element contains 2 atoms of hydrogen. 2 H represents 2 separate atoms of hydrogen; H 2 represents 1 molecule of hydrogen and 2H 2 represents 2 molecules of hydrogen.
What is the formula for hydrogen?
One molecule of hydrogen element contains two atoms of hydrogen, therefore, the formula of hydrogen is H 2. For elements that exist as atoms, their chemical formulae represent their atoms. For elements that exist as molecules, their chemical formulae represent their molecules. Subscript number at the right bottom shows the number ...
What are ionic compounds?
Ionic compounds consist of cations and anions. (a) Cations are positively-charged ions. (b) Anions are negatively-charged ions. To construct chemical formulae of ionic compounds, we need to know the formulae of cations and anions. Below tables show the formulae of some common cations and anions.
What does formula mean in math?
Formula gives the name of all the elements present in the molecule. Formula gives the number of atoms of each element present in one molecule. Formula represents a definite mass of the substance.
Why are ionic compounds neutral?
This is because the total positive charges are equal to the total negative charges. Steps in constructing the chemical formula of an ionic compound: From its name, write the formulae of its cation and anion.
What are Compounds?
A compound is a pure substance that is formed by the chemical combination of two or more elements in a fixed proportion by mass. Compound formation always takes place as a result of a chemical reaction. Therefore, a compound does not exhibit the properties of its constituent elements. On breaking down the compound, the smallest entity so obtained is called a molecule of the compound.
Why do compounds have a molecular formula?
Solution: Compounds are pure substances whereas mixtures are impure. A compound always has a fixed chemical composition by mass, which means that the elements that combine together to form a compound always do so in a fixed ratio. This is why compounds have a molecular formula. Compounds have fixed melting and boiling points. They do not show the properties of their constituent elements. A compound cannot be broken down into its elements by simple physical methods.
How are covalent compounds formed?
They are also called molecular compounds. They are formed as a result of the sharing of electrons between two or more non-metals.
What are the physical properties of non-metals?
The physical properties of non-metals include brittleness, comparatively lower tensile strength, non-luster, non-conductivity or insulation, lower melting and boiling points, etc.
What are impure substances?
Pure substances can further be classified into elements and compounds. Impure substances are also called mixtures. Depending on their composition, they can further be classified into homogeneous and heterogeneous.
What is an ionic compound?
Ionic compounds are formed between a metal and a non-metal. They are also called electrovalent compounds as they involve ions. The metal atom loses electrons to form a cation, and the non-metal atom gains the electron to form an anion.
Which element has the tendency to lose electrons in order to gain stability?
Metals are the elements that have the tendency of losing electrons in order to gain stability, that is, they exhibit electropositivity. The physical properties of metals include hardness, high tensile strength, luster, conductivity, high melting and boiling points, etc.

What Is An element?
Classification of Elements
- Depending on the properties, elements can be classified into metals, nonmetals, and metalloids. Metals are the elements that have the tendency of losing electrons in order to gain stability, that is, they exhibit electropositivity. The physical properties of metals include hardness, high tensile strength, luster, conductivity, high melting and boil...
What Are compounds?
- A compound is a pure substance that is formed by the chemical combination of two or more elements in a fixed proportion by mass. Compound formation always takes place as a result of a chemical reaction. Therefore, a compound does not exhibit the properties of its constituent elements. On breaking down the compound, the smallest entity so obtained is called a molecule …
Classification of Compounds
- On the basis of the formation, compounds can be classified as ionic compounds and covalent compounds. Ionic compounds are formed between a metal and a non-metal. They are also called electrovalent compounds as they involve ions. The metal atom loses electrons to form a cation, and the non-metal atom gains the electron to form an anion. Examples include sodium chloride, …
About The Chapter
- Elements and compounds form the foundation of chemistry. Without these, two chemistry is far more incomplete. This basic foundation of chemistry is introduced to students in the ninth grade and as discussed briefly in chapter 2 called Is Matter around us pure? Just stop explaining in-depth about what are the types of mixtures around us and what are the things that form a mixtur…
Study with Vedantu
- The Vedantu team has made the learning process much easier for students who may find it difficult to grasp the concept of elements and compounds as the study notes provided by Vedantu are written in an extremely simplified manner. As the main objective of producing these study notes is to help students get a clear knowledge of the basic foundations of chemistry so t…
Key Concepts Needed to Understand Elements and Compounds
- What is A Mixture?
- Types of Mixtures
- What is A Solution?
- Properties of A Solution