
What is the difference between complete and incomplete combustion reactions?
Combustion reactions can be of two types depending on the amount of oxygen that is available for the reaction. The difference between Complete Combustion and Incomplete Combustion is the amount of oxygen available for it.
What happens if the combustion has not completely happened?
If the combustion had not happened completely, carbon monoxide and other particles released into the atmosphere and can cause a lot of pollution. What is the Difference Between Complete and Incomplete Combustion?
Does the incomplete combustion of a hydrocarbon produce carbon monoxide?
If anyone tells you that the “incomplete combustion” of a hydrocarbon produces only carbon monoxide, they don’t have a firm grasp of what is actually I assume you are referring to the complete combustion of a hydrocarbon which produces carbon dioxide and water vapor. We don’t write equations for the “incomplete combustion.”
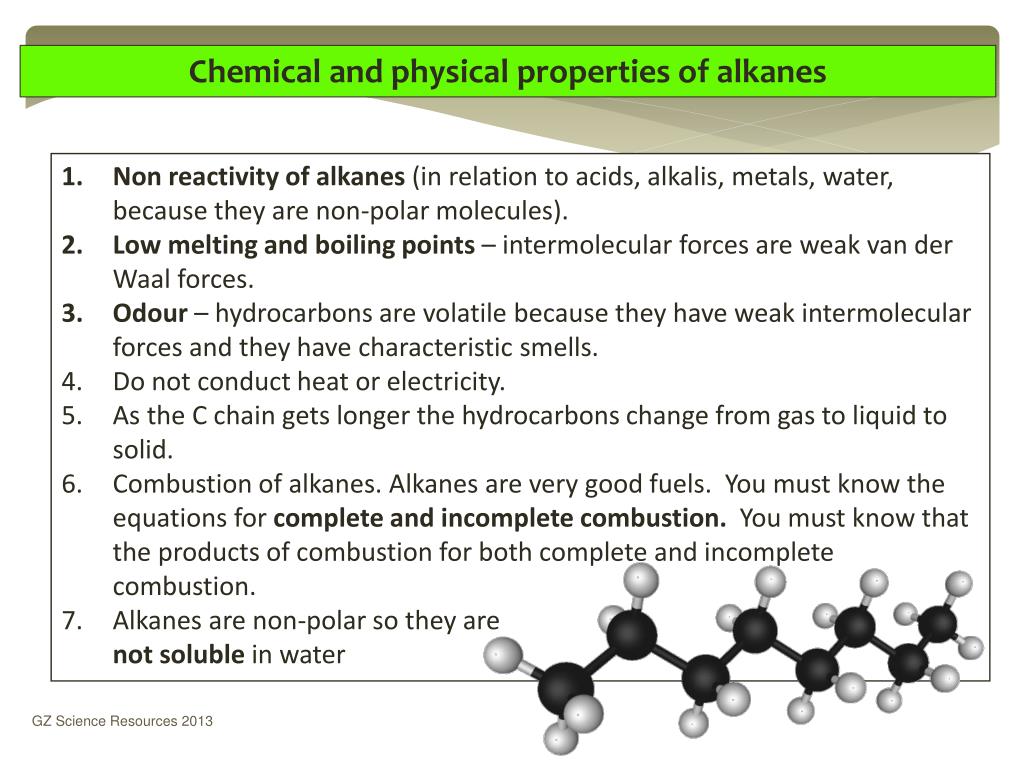
What are the products of incomplete combustion?
But the amount of energy released from this combustion is comparatively low. The major products of incomplete combustion include Carbon monoxide (CO), carbon dust (we call it “soot”) and water (H 2 O). The general formula for incomplete combustion is shown below.
How do you know if its a complete or incomplete combustion?
Complete combustion takes place in the presence of a sufficient amount of oxygen while an incomplete combustion reaction takes place when there is an insufficient amount of oxygen supply.
What are the signs of complete combustion?
Good signs that you're dealing with a combustion reaction include the presence of oxygen as a reactant and carbon dioxide, water, and heat as products. Inorganic combustion reactions might not form all of those products but remain recognizable by the reaction of oxygen.
How do you test for complete combustion?
Carbon dioxide gas turns limewater from colourless to milky white. In the above experiment, the limewater in the boiling tube changes colour indicating carbon dioxide is produced by combustion of the hydrocarbon fuel. If water is produced it will condense in the u tube cooled by the ice water.
What does incomplete combustion look like?
Incomplete combustion is often indicated by the production of a yellow or orange colored flame. Not all of the reactants are consumed in incomplete combustion with the result that less energy is produced at the end of this reaction when compared with complete combustion.
What is formed in incomplete combustion?
During incomplete combustion part of the carbon is not completely oxidized producing soot or carbon monoxide (CO). Incomplete combustion uses fuel inefficiently and the carbon monoxide produced is a health hazard.
What is the result of complete combustion?
Complete combustion will produce only carbon dioxide and water as the products and nothing will be leftover. Incomplete combustion will produce other byproducts like carbon monoxide or carbon soot left behind. There are instruments available that can measure the amount of carbon dioxide and water vapor produced in an enclosed chamber.
What is combustion in education?
Combustion is the burning of hydrocarbons (compounds containing carbon, hydrogen, and possibly some oxygen) in the presence of oxygen to produce water and carbon dioxide.
What is the process of burning hydrocarbons?
Combustion is the burning of hydrocarbons (compounds containing carbon, hydrogen, and possibly some oxygen) in the presence of oxygen to produce water and carbon dioxide. Combustion requires three things to occur: fuel (hydrocarbons), oxygen (from the air), and a catalytic spark.
What is the difference between complete combustion and incomplete combustion?
The difference between complete and incomplete combustion is that complete combustion takes place when there is a constant and enough oxygen supply whereas incomplete combustion takes place when there isn’t enough oxygen supply.
What happens to the flame during complete combustion?
Figure 01: Flame during Complete Combustion. In the reaction, the oxidant oxidizes the fuel. So this is an oxidation reaction. When we use hydrocarbon fuels, the products after complete combustion are usually carbon dioxide and water. In complete combustion, few products forms, and it gives the maximum energy output that the reactant can give.
What is the form of combustion that takes place when there is a constant and enough oxygen supply?
Complete combustion is in the form of combustion that takes place when there is a constant and enough oxygen supply. Incomplete combustion is the form of combustion that takes place when there isn’t enough oxygen supply.
What happens when a hydrocarbon undergoes complete combustion?
Furthermore, if a hydrocarbon undergoes complete combustion, it produces only carbon dioxide and water while carbon monoxide and carbon particles may form in incomplete combustion.
What happens when there isn't enough oxygen in the atmosphere?
If the combustion had not happened completely, carbon monoxide and other particles released into the atmosphere and can cause a lot of pollution .
What is the reaction of oxygen and another substance?
There, oxygen combines with another molecule, to produce an oxide. In this reaction, oxygen undergoes reduction, and the other substance undergoes oxidation. So basically oxidation reaction is adding oxygen to another substance. Another way to describe oxidation is as loss of hydrogen.
Does incomplete combustion cause pollution?
Moreover, Complete combustion does not cause environmental pollution. However, incomplete combustion cause environmental pollution .
What is the difference between complete combustion and incomplete combustion?
Main Differences Between Complete Combustion and Incomplete Combustion 1 The main difference between Complete Combustion and Incomplete Combustion reactions is the amount of oxygen. If the amount is sufficient or more, it will be a complete reaction, and if it is less than sufficient, it will be an incomplete reaction. 2 Complete Combustion reactions are usually characterized by a blue and smoke-less flame. Incomplete Combustion reactions are characterized by a sooty, smokey yellow flame. 3 The products of Complete reaction primarily include carbon dioxide (CO2) and water (H2O). Incomplete reactions produce carbon monoxide (CO) and water (H2O). 4 The products of a complete combustion reaction are environment friendly and do not cause pollution whereas the products of an Incomplete Combustion reaction are major pollutants in today’s world. 5 A complete combustion reaction produces more energy than an Incomplete Combustion reaction with the same product. This is because a complete combustion reaction utilizes the whole product, whereas Incomplete combustion utilizes only a part of it.
What is incomplete combustion?
Incomplete Combustion. Definition. The combustion reaction which takes place in the presence of a sufficient or abundant amount of oxygen. Also known as Complete Combustion. The combustion reaction that takes place in the presence of an insufficient amount of oxygen. Flame-type.
What is the reaction of a flame with no smoke?
When the amount of oxygen is insufficient for the process of combustion, then the reaction is known as an Incomplete Combustion reaction. This reaction is usually accompanied by a yellow sooty flame.
What is the energy released in combustion?
Combustion reactions release energy in the form of heat and light. Please note that it is necessary for a combustion reaction for oxygen (O2). However, the quantity of oxygen present in the reaction may vary depending on the other reactants and other environmental factors. An example of a combustion reaction involving Propane and oxygen is as below:
Which reaction produces more energy, complete combustion or incomplete combustion?
A complete combustion reaction produces more energy than an Incomplete Combustion reaction with the same product. This is because a complete combustion reaction utilizes the whole product, whereas Incomplete combustion utilizes only a part of it.
Is carbon monoxide a complete combustion reaction?
However, it is to be noted that for this reaction to be a Complete Combustion reaction, there should not be a single molecule of carbon monoxide. A very common example of Incomplete Combustion is the burning of coal. This produces a lot of soot and smoke and hence causes a lot of environmental degradation.

What Is Complete Combustion?
What Is Incomplete Combustion?
- When there isn’t enough oxygen, incomplete combustion takes place. If the combustion had not happened completely, carbon monoxide and other particles released into the atmosphere and can cause a lot of pollution.
What Is The Difference Between Complete and Incomplete Combustion?
- Complete combustion is in the form of combustion that takes place when there is a constant and enough oxygen supply. Incomplete combustion is the form of combustion that takes place when there isn’t enough oxygen supply. In complete combustion, a limited number of products form while in incomplete combustion, some products may form. Furthermore, if a hydrocarbon under…
Summary – Complete vs Incomplete Combustion
- Combustion may take place in two types as complete and incomplete combustion. Both are oxidation reactions. The difference between complete and incomplete combustion is that complete combustion takes place when there is a constant and enough oxygen supply whereas incomplete combustion takes place when there isn’t enough oxygen supply. 1. “GCSE Bite...
Complete Combustion vs Incomplete Combustion
- The main difference between Complete Combustion and Incomplete Combustion is the amount of oxygen available for it. If the amount is sufficient or more, it is a Complete Combustion reaction and if it is less, it is an Incomplete Combustion reaction. When there is a sufficient or an abundant amount of oxygen available during the process of combustio...
What Is Complete Combustion?
- Complete combustion is the combustion process where the amount of oxygen involved in the relationship is sufficient in amount or more than required. oxygen plays the role of an oxidizing agent in this case. Usually, these reactions take place with hydrocarbons being on the reactant side as reducing agents. Hydrocarbons and oxygen react together to form waterand carbon diox…
What Is Incomplete Combustion?
- An Incomplete Combustion reaction is a reaction where the amount of oxygen present in the reaction is insufficient then the required amount of oxygen needed to carry out the reaction in a complete manner. Like Complete Combustion reactions, the reactants play the same role, where oxygen is an oxidizing agent, and the hydrocarbons are reducing agents. This type of reaction is …
Main Differences Between Complete Combustion and Incomplete Combustion
- The main difference between Complete Combustion and Incomplete Combustion reactions is the amount of oxygen. If the amount is sufficient or more, it will be a complete reaction, and if it is less t...
- Complete Combustion reactions are usually characterized by a blue and smoke-less flame. Incomplete Combustion reactions are characterized by a sooty, smokey yellow flame.
- The main difference between Complete Combustion and Incomplete Combustion reactions is the amount of oxygen. If the amount is sufficient or more, it will be a complete reaction, and if it is less t...
- Complete Combustion reactions are usually characterized by a blue and smoke-less flame. Incomplete Combustion reactions are characterized by a sooty, smokey yellow flame.
- The products of Complete reaction primarily include carbon dioxide (CO2) and water (H2O). Incomplete reactions produce carbon monoxide (CO) and water (H2O).
- The products of a complete combustion reaction are environment friendly and do not cause pollution whereas the products of an Incomplete Combustion reaction are major pollutants in today’s world.
Conclusion
- Combustion Reactions are an essential part of our day to day lives and have a pivotal role in nature. Both the types of combustion reactions discussed above are important for us, although a complete combustion reaction might seem to be more preferable than Incomplete Combustion reactions, as they give us the maximum amount of energy possible without any environmental p…
References