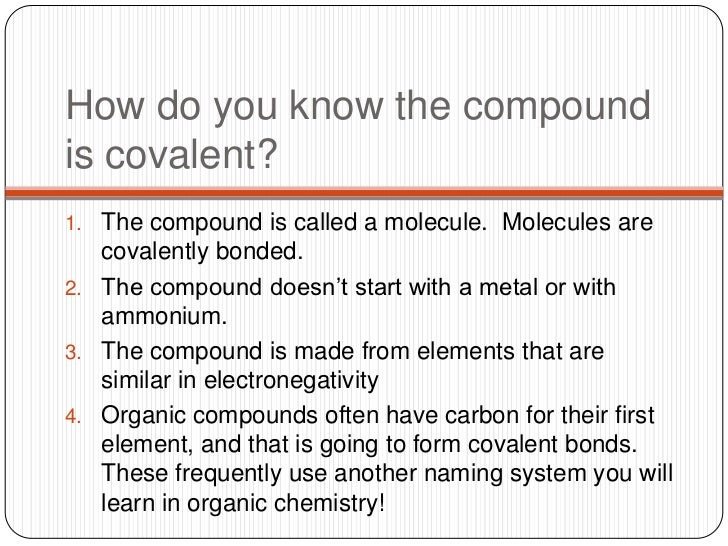
A dead giveaway the compound contains both bonds is when it has a metal cation bonded to an anion that only contains nonmetals. Also, any compound that contains the ammonium
Ammonium
The ammonium cation is a positively charged polyatomic ion with the chemical formula NH⁺₄. It is formed by the protonation of ammonia. Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations, where one or mo…
Covalent bond
A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding.
What are 5 examples of ionic compounds?
Ionic bond examples include:
- LiF - Lithium Fluoride
- LiCl - Lithium Chloride
- LiBr - Lithium Bromide
- LiI - Lithium Iodide
- NaF - Sodium Fluoride
- NaCl - Sodium Chloride
- NaBr - Sodium Bromide
- NaI - Sodium Iodide
- KF - Potassium Fluoride
- KCl - Potassium Chloride
What are 5 examples of covalent bonds?
What are 5 examples of covalent bonds? Hydrogen (H 2) Hydrogen (H) is the simplest of all elements. … Oxygen (O 2) The valency of oxygen (O) is two, which means that it requires two electrons to complete its outermost (valence) shell. … Nitrogen (N 2) … Water (H 2 O) … Carbon Dioxide (CO 2) … Methane (CH 4) … Ammonia (NH 3) …
What is difference between covalent and ionic?
The main difference between covalent and ionic bonds is that ionic bonds occur between two species which are electrostatically attracted towards each other, whereas covalent bonds occur covalently through the sharing of electrons between their outer shells. In general, metallic elements tend to form ionic bonds, and non-metallic elements tend to form covalent bonds.
What are common ionic bonds?
Many common ionic compounds contain polyatomic ions, such as these ionic compound examples:
- Antacids - are used to combat heartburn and contain magnesium hydroxide, Mg (OH) 2, an ionic compound.
- Chalk - blackboard chalk contains calcium carbonate, CaCO 3.
- Baking soda - this is the common name for sodium bicarbonate, NaHCO 3. ...
- A common fertilizer, ammonium nitrate, NH 4 NO 3 - this is composed of two polyatomic ions.

How do you tell if a compound contains both ionic and covalent bonds?
A compound can have both ionic and covalent bonds if the covalent bonds are part of a polyatomic ion. Examples of polyatomic ions are ammonium ion, NH4+, sulfate ion, SO42-, hypochlorite ion, and ClO-.
What contains both ionic and covalent bonds?
Answer - The compound which contains both ionic and covalent bond is KCN (Potassium Cyanide). These substances include polyatomic ions. Numerous of these compounds also contain hydrogen, a nonmetal, and a metal.
What is the difference between ionic and covalent bonds?
Remember, an ionic bond occurs when one atom essentially donates a valence electron to another atom. A covalent bond involve s atoms sharing electro ns. In pure covalent bonds, this sharing is equal. In polar covalent bonds, the electron spends more time with one atom than the other. KCN – potassium cyanide.
Why use a table to find electronegativity?
The table is great for identifying the type of bond within the cation and the anion when polyatomic ions occur .
What are the bonds between carbon and nitrogen?
For example, in potassium cyanide (KCN), the carbon (C) and nitrogen (N) are both nonmetals, so they share a covalent bond. The potassium atom (K) is a metal, so it bonds to the nonmetallic anion via an ionic bond. X-ray diffraction of KCN crystals verifies this arrangement. The potassium ions are separate from the bonded carbon and nitrogen ions that form the cyanide anion. Compounds with both ionic and covalent bonds form ionic crystals. When these compounds melt or dissolve into water, the ionic bonds break, but the covalent bonds remain intact. In a melted compound, the cation and anion remain attracted to one another, but not enough to organize into a crystal.
How to tell if a chemical bond is covalent or nonpolar?
Usually, all you have to do to predict the type of chemical bond between two atoms is compare their electronegativity values. Nonpolar covalent bond – If the atoms are identical, there is no electronegativity difference and the bond is covalent.
What is the X-ray diffraction of KCN crystals?
X-ray diffraction of KCN crystals verifies this arrangement. The potassium ions are separate from the bonded carbon and nitrogen ions that form the cyanide anion. Compounds with both ionic and covalent bonds form ionic crystals. When these compounds melt or dissolve into water, the ionic bonds break, but the covalent bonds remain intact.
What type of bond is formed between nonmetals?
Polar covalent bond – The electronegativity difference is between 0.4 and 1.7. This is the type of bond formed between most nonmetals. Ionic bond – The electronegativity difference is greater than 1.7. You can use a table to see electronegativity values of atoms.
Which compound has ionic and covalent bonds?
Also, any compound that contains the ammonium (NH4 +) cation has both ionic and covalent bonds. The nitrogen and hydrogen atoms are joined by covalent bonds. The polyatomic cation is highly electropositive, so it forms ionic bonds with any anion.
How to determine if a compound is ionic or covalent?
Assuming the molecular compound is known, this is a fairly easy deducation to make: 1 If there exists a bond between two non-metals, there is most likely a covalent bond involved (e.g. H2O, where hydrogen and oxygen are non-metal) 2 If there exists a bond between a metal and non-metal, there is most likely an ionic bond involved (e.g. NaCl, where sodium is a metal and chlorine is non-metal) 3 If there exists within the compound both bonds described above, then ionic and covalent are involved (e.g. NaOH, where sodium is a metal and hydroxide is considered a non-metal, whilst within hydroxide
What is CaCO3 and CaSO4?
CaCO3 : Calcium sulfite, or calcium sulphite. CaSO4: Calcium sulfate (or calcium sulphate ). Also remember also polyatomic ions ( relating to a molecule made up of more than two atoms.) are held together by covalent bonds, so this compound contains both ionic and covalent bonds. Related Answer.
What are the constituents of a polyatomic ion?
Thus, carbonates, nitrites, nitrates, sulphites, sulphates, phosphates, chlorates, acetates etc. of metals have both ionic and covalent bonds. The hydroxides of metals, which are commonly termed bases, also have both type of bonding as within the hydroxide ion (OH-) ...
Which type of bond holds polyatomic ions together?
Also remember also polyatomic ions ( relating to a molecule made up of more than two atoms.) are held together by covalent bonds, so this compound contains both ionic and covalent bonds.
What type of bond forms between elements with a smaller difference in electronegativity?
Covalent bond forms between elements which have smaller difference between their electronegativity. For example when there are two non metals.
How to determine bonding nature?
1- Every compound that are formed between metal atom and non metal atom are ionic in nature. 2- Every compound that are formed between non metals atoms are covelent in nature.
What type of bond is formed when ions are oppositely charged?
Ionic bond, also known as electrovalent bond, is a type of bond formed from the electrostatic attraction between oppositely charged ions in a chemical compound. These kinds of bonds occur mainly between a metallic and a non metallic atom.