
Precipitation reactions occur when cations and anions in aqueous
Water
Water is a transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's streams, lakes, and oceans, and the fluids of most living organisms. It is vital for all known forms of life, even though it provides no calories or organic nutrients. Its che…
How do you know if a precipitation reaction has occurred?
How do you tell if a precipitation reaction will occur? If the rules state that an ion is soluble, then it remains in its aqueous ion form. If an ion is insoluble based on the solubility rules, then it forms a solid with an ion from the other reactant. If all the ions in a reaction are shown to be soluble, then no precipitation reaction occurs.
How do you predict precipitation reactions?
Predicting Precipitation Reactions
- Precipitation Reactions. Sometimes ions in solution react with each other to form a new substance that precipitates; this reaction is called a precipitation reaction.
- Rules of Precipitation. Note that soluble compounds will dissolve in water and insoluble compounds will not. ...
- Example of Precipitation Reaction. ...
What happens during a precipitation reaction?
Precipitation reactions are usually double displacement reactions involving the production of a solid form residue called the precipitate. These reactions also occur when two or more solutions with different salts are combined, resulting in the formation of insoluble salts that precipitate out of the solution.
Which chemical equation represents a precipitation reaction?
This reaction can be also be written in terms of the individual dissociated ions in the combined solution. This is known as the complete ionic equation: Ag + (aq) + NO 3−(aq) + K + (aq) + Cl −(aq) → AgCl (s) + K + (aq) + NO 3−(aq) A final way to represent a precipitation reaction is known as the net ionic equation.

How can we identify precipitation method?
Identifying a Precipitation Reaction Identify the reactants and products. Step 2: Determine if two aqueous compounds are on the reactants side of the reaction, and that an insoluble salt is on the products side of the reaction. Step 3: Make sure the chemical reaction is correctly balanced.
What is a precipitation reaction example?
One of the best examples of precipitation reactions is the chemical reaction between potassium chloride and silver nitrate, in which solid silver chloride is precipitated out. This is the insoluble salt formed as a product of the precipitation reaction.
How do you test for precipitation in chemistry?
Precipitate testsdissolve a small quantity of the substance in water.place about 5cm 3 of the solution into a test tube.add a few drops of sodium hydroxide solution.record the colour of any precipitate that's formed.add dilute sodium hydroxide solution until it is in excess and record the result.
What kind of reaction is precipitation?
A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and are sometimes referred to as double displacement, double replacement, or metathesis reactions.
What are 2 examples of precipitate?
AgNO3(aqueous) + KCl(aqueous) —–AgCl(precipitate) + KNO3(aqueous)AgNO3(aqueous) + NaCl(aqueous) —- AgCl↓ + NaNO3 (aqueous)Mg(OH)2(s) + 2HCl (aqueous) ——— MgCl2(aqueous) + 2H2O(l)
What are the three examples of precipitation?
The different types of precipitation are:Rain. Most commonly observed, drops larger than drizzle (0.02 inch / 0.5 mm or more) are considered rain. ... Drizzle. Fairly uniform precipitation composed exclusively of fine drops very close together. ... Ice Pellets (Sleet) ... Hail. ... Small Hail (Snow Pellets) ... Snow. ... Snow Grains. ... Ice Crystals.
Why do precipitation reactions occur?
When a solution containing a particular cation (a positively charged ion) is combined with another solution containing a certain anion (a negativel...
Is precipitation a sign of a chemical reaction?
The formation of a precipitate also suggests the presence of a chemical reaction. When a silver nitrate solution is poured into a sodium chloride s...
What is an example of formation of precipitate?
When a silver nitrate solution is poured into a sodium chloride solution, a chemical reaction occurs, producing a white silver chloride precipitate...
Is Salt a precipitate?
The insoluble salt falling out of the solution is referred to as the precipitate, hence the name of the reaction. Precipitation reactions in the so...
What factors affect precipitation?
Prevailing waves, the presence of mountains, and seasonal waves are the 3 major factors that influence precipitation. Mountain ranges are a series...
What is precipitation reaction?
Precipitation reactions are usually double displacement reactions involving the production of a solid form residue called the precipitate. These reactions also occur when two or more solutions with different salts are combined, resulting in the formation of insoluble salts that precipitate out of the solution.
What is the white precipitate in a precipitation reaction?
In the above reaction, a white precipitate called silver chloride or AgCl is formed which is in the solid-state. This solid silver chloride is insoluble in water. Precipitation reactions help in determining the presence of different ions present in a particular solution. The other example of a precipitation reaction is the reaction between calcium ...
Why is precipitation important?
Precipitation reaction helps in determining a particular element present in the given solution. These reactions also monitor the formation of a precipitate when some chemical is added to solutions. These are used for the extraction of magnesium from the seawater. The human body also encounters these reactions existing between antigens and antibodies.
What is the reaction between calcium chloride and potassium hydroxide?
The other example of a precipitation reaction is the reaction between calcium chloride and potassium hydroxide, resulting in the formation of an insoluble salt Called calcium hydroxide. The chemical equation for this reaction is below-
What state does precipitation occur in?
The precipitation reaction undergoes in aqueous solutions or medium in an ionic state.
What is the purpose of chemical equations in precipitation reactions?
Chemical equations can help us understand the chemical reactions between various elements or compounds. Chemical equations show the reactants and the products that are involved in these reactions.
What are some examples of chemical reactions?
Many chemical reactions occur in our daily lives. Common examples of such reactions are burning, corrosion, cooking of food and digestion . One important class of chemical reactions are precipitation reactions. In such reactions, two different soluble salts (which are in aqueous solutions) combine to form two products.
How do precipitation reactions occur?
Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Whether or not such a reaction occurs can be determined by using the solubility rules for common ionic solids. Because not all aqueous reactions form precipitates, one must consult the solubility rules before determining the state of the products and writing a net ionic equation. The ability to predict these reactions allows scientists to determine which ions are present in a solution, and allows industries to form chemicals by extracting components from these reactions.
What determines if a reaction forms a precipitate?
These rules provide guidelines that tell which ions form solids and which remain in their ionic form in aqueous solution. The rules are to be followed from the top down, meaning that if something is insoluble (or soluble) due to rule 1, it has precedence over a higher-numbered rule.
What are precipitates in chemistry?
Precipitates are insoluble ionic solid products of a reaction, formed when certain cations and anions combine in an aqueous solution. The determining factors of the formation of a precipitate can vary. Some reactions depend on temperature, such as solutions used for buffers, whereas others are dependent only on solution concentration. The solids produced in precipitate reactions are crystalline solids, and can be suspended throughout the liquid or fall to the bottom of the solution. The remaining fluid is called supernatant liquid. The two components of the mixture (precipitate and supernate) can be separated by various methods, such as filtration, centrifuging, or decanting.
What is the reaction that occurs when cations and anions in aqueous solution combine to form an in?
Contributors and Attributions. Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Whether or not such a reaction occurs can be determined by using the solubility rules for common ionic solids.
What are spectator ions in ionic equations?
Lastly, eliminate the spectator ions (the ions that occur on both sides of the equation unchanged). In this case, they are the sodium and chlorine ions . The final net ionic equation is:
How to predict precipitate?
The key to predicting a precipitate is to learn the solubility rules. Pay particular attention to compounds listed as "slightly soluble" and remember that temperature affects solubility. For example, a solution of calcium chloride is typically considered soluble in water, yet if the water is cold enough, the salt doesn't readily dissolve. Transition metal compounds may form a precipitate under cold conditions, yet dissolve when it's warmer. Also, consider the presence of other ions in a solution. This can affect solubility in unexpected ways, sometimes causing a precipitate to form when you didn't expect it.
What is a precipitate in chemistry?
A precipitate will form if the resulting compound is insoluble in water. For example, a silver nitrate solution (AgNO 3) is mixed with a solution of magnesium bromide (MgBr 2 ). The balanced reaction would be:
What is the finished reaction?
The finished reaction is: The solubility rules are a useful guideline to predict whether a compound will dissolve or form a precipitate. There are many other factors that can affect solubility, but these rules are a good first step to determine the outcome of aqueous solution reactions.
When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce answer?
When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. This guide will show how to use the solubility rules for inorganic compounds to predict whether or not the product will remain in solution or form a precipitate . Aqueous solutions of ionic compounds are comprised ...
Can transition metals form precipitate?
Transition metal compounds may form a precipitate under cold conditions, yet dissolve when it's warmer. Also, consider the presence of other ions in a solution. This can affect solubility in unexpected ways, sometimes causing a precipitate to form when you didn't expect it.
How are precipitates formed?
Most precipitates are formed in a double-replacement reaction. A double-replacement reaction is when the ions in two compounds exchange places with each other in an aqueous solution. AX + BY --> AY + BX, where A, B, X and Y are all ions. An ionic solution is when the ions of a compound have dissociated in an aqueous solution.
What is the difference between a precipitate and an ionic solution?
A precipitate is a solid formed when the cation and anion in an aqueous solution react to form an insoluble compound. An ionic solution is when the ions of a compound have dissociated in an aqueous solution. A net ionic equation is an equation written so that only the substances that are participating in the reaction are shown.
What is the reaction between two aqueous solutions?
When two aqueous solutions react, they sometimes form solids in the solution. The solid is called a precipitate. Precipitation reactions occur when the cations of one reactant and the anions of a second reactant found in aqueous solutions combine to form an insoluble ionic solid that we call a precipitate.
What compound combine to form a precipitate?
Notice we combined the potassium and the nitrate into a soluble compound. Lead and iodide combine to form a precipitate. We always balance the equation too.
What is the final product of a precipitate?
You can see that the final product is a solid. This is the precipitate that is forming. In this reaction, it is silver chloride.
How to write a net ionic equation?
So, you start with the reaction of: KCl (aq) + AgNO 3 (aq) --> KNO 3 (aq) + AgCl (s)
What is precipitation reaction?
Precipitation reactions typically involve the precipitation of an insoluble precipitate. Ions in solution can form insoluble compounds, and precipitate (i.e. deposit) out of solution. Inorganic chemistry is rich in precipitation reactions: Such precipitation reactions can be used to effect separations in mixtures, ...
What is the reaction of acid and base?
And acid-base reactions are reactions which are typically conducted in water. The characteristic cation of the solution, the acid, which is water we may represent as hydronium ion, H 3O+, reacts with the characteristic anion of the solution, the base, which in water we represent as hydroxide ion, H O−, to form water:
Is a redox reaction a formal change?
Redox reactions involve a formal change in oxidation number. The combustion of hydrocarbons is certainly an oxidation reaction, as carbon, in the hydrocarbon, is oxidized to CO2, in which carbon has a formal oxidation number of C( +I V).
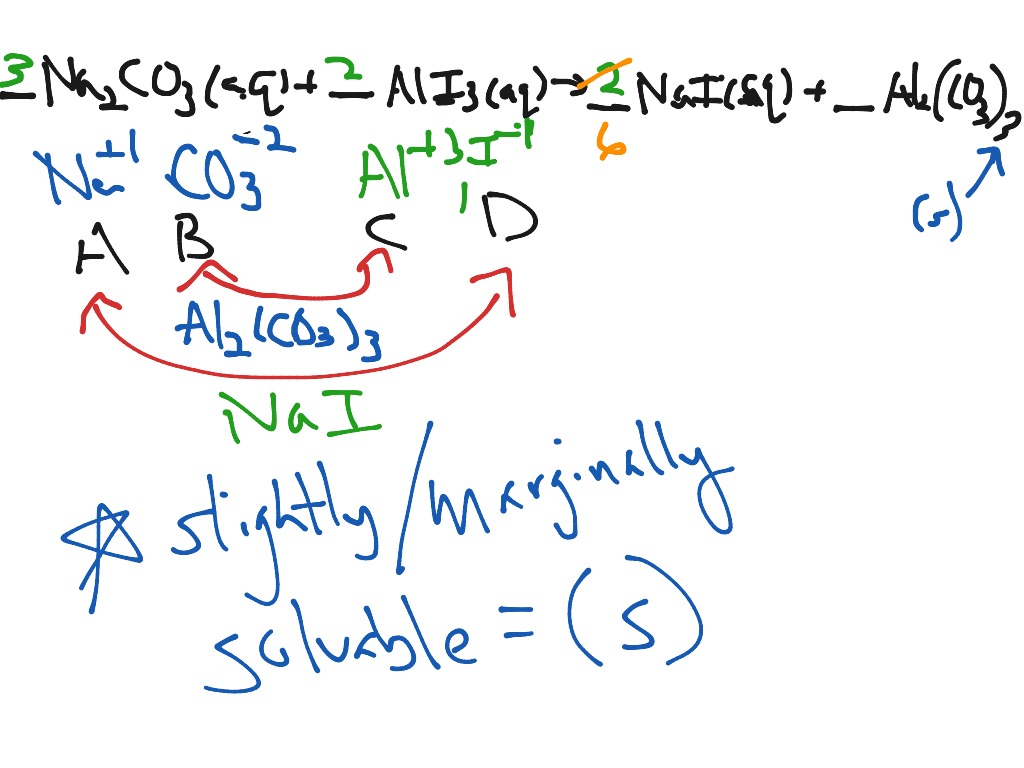
Identifying A Precipitation Reaction Vocabulary
- Chemical Reaction:A chemical reaction is a change that occurs on a molecular level where an initial substance(s), commonly known as a reactant(s), is converted into a new substance(s), known as a product(s). Precipitation Reaction:A precipitation reaction is a chemical reaction whe…
Identifying A Precipitation Reaction Example: Precipitation of Silver Chloride
- Bill is conducting an electrochemistry experiment but he has run out of silver chloride (AgCl) and needs to synthesize more of the compound. He has silver nitrate ({eq}AgNO_3{/eq}) and sodium chloride (NaCl) on hand. Which option below correctly represents the reaction Bill will use to make more silver chloride. A.{eq}AgNO_3 (l) + NaCL (s) \rightarrow AgCl (s) + NaNO_3 (aq){/eq} B.{e…
Identifying A Precipitation Reaction Example: Precipitation of Calcium Carbonate
- The following reaction represents the precipitation of Calcium Carbonate ({eq}CaCO_3{/eq}) True or False? {eq}Ca(OH)_2 (aq) + H_2 CO_3 (aq) = H_2 O (l) + CaCO_3 (s){/eq} Step 1:Read through the given information in the problem for the chemical reaction and identify the reactants and products. We are trying to precipitate calcium carbonate which we can see that it is in fact o…