Formation of an acetal occurs when the hydroxyl group of a hemiacetal becomes protonated and is lost as water. The carbocation that is produced is then rapidly attacked by a molecule of alcohol. Loss of the proton from the attached alcohol gives the acetal.
What is acetal material?
Acetal (Copolymer) is an engineering thermoplastic polymer that provides good strength and stiffness. Commonly known as Delrin® or Celcon® or Acetron®, it is easily machined with conventional metal working equipment and can be machined to very close tolerances. Both sheet and rod are available in many thicknesses and diameters.
How do you make acetals with orthoesters?
Acetal. A way to improve this is to use an orthoester as a source of alcohol. Aldehydes and ketones undergo a process called acetal exchange with orthoesters to give acetals. Water produced along with the acetal product is used up in hydrolysing the orthoester and producing more alcohol to be used in the reaction.
How do you convert hemiacetal to acetal?
Formation of an acetal occurs when the hydroxyl group of a hemiacetal becomes protonated and is lost as water. The carbocation that is produced is then rapidly attacked by a molecule of alcohol. Loss of the proton from the attached alcohol gives the acetal. Aldehyde to acetal conversion. Ketone to ketal conversion.
How can I convert an aldehyde to an acetal?
An undergraduate or graduate student in Chemistry may indeed carry out the above reaction in a round bottom flask using Methanol as the solvent in order to convert an aldehyde to an acetal as part of a Protecting Group strategy.

How many steps is acetal formation?
seven distinct stepsLesson Summary. Acetals are diether products made from the acid catalyst synthesis of an aldehyde or ketone with two alcohol molecules. The mechanism of forming an acetal involves seven distinct steps.
What makes a compound acetal?
What is Acetal? “An acetal is an organic molecule where two separate oxygen atoms are single bonded to a central carbon atom.” Two distinct oxygen atoms are singly linked to a central carbon atom in an acetal, which is an organic molecule. R2C(OR')2 is the general structure of acetals.
Can you make an acetal from an aldehyde?
Voiceover: If we react an aldehyde, or a ketone, with an excess of alcohol, in an acidic environment, we are going to form an acetal.
How do you convert hemiacetal to acetal?
Mechanism for Hemiacetal and Acetal Formation Further protonation of the OH group in the hemiacetal allows for the elimination of water to form an oxonium ion. A second alcohol nucleophile adds to the oxonium ion to produce a protonated acetal. After deprotonation, the product acetal is formed.
What is acetal material?
Acetal (polyoxymethylene or POM) is a high strength, low friction engineering plastic that has excellent wear properties in both wet and dry environments. Acetal is chemically resistant to hydrocarbons, solvents, and neutral chemicals.
What is acetal give example?
Acetal Examples Dimethoxymethane is an acetal compound. Acetal is also a common name for the compound 1,1-diethoxyethane. The compound polyoxymethylene (POM) is a plastic that is also called simply "acetal" or "polyacetal."
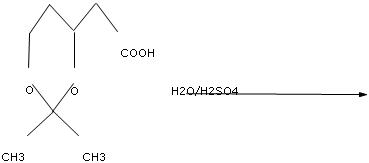
How the conversion of an aldehyde to acetal can carried out?
Introduction. It has been demonstrated that water adds rapidly to the carbonyl function of aldehydes and ketones to form geminal-diol. In a similar reaction alcohols add reversibly to aldehydes and ketones to form hemiacetals (hemi, Greek, half). This reaction can continue by adding another alcohol to form an acetal.
Which two reagent are used in the preparation of acetal?
Acetals are geminal-diether derivatives of aldehydes or ketones, formed by reaction with two equivalents (or an excess amount) of an alcohol and elimination of water.
How do you identify acetal?
3:4611:46Acetal Ketal Hemiacetal Hemiketal Reaction Overview and ShortcutYouTubeStart of suggested clipEnd of suggested clipWhen you have a carbon that has a hydrogen and two o R groups attached. That's how you recognize anMoreWhen you have a carbon that has a hydrogen and two o R groups attached. That's how you recognize an acetone. If you have a carbon with two R groups attached.
What's the difference between hemiacetal and acetal?
Hemiacetal is formed as an intermediate product between acetal formation. Hemiacetal and acetal are acknowledged as functional groups. The critical difference between hemiacetal and acetal is that hemiacetal contains one -OH and one -OR group while acetal contains two -OR groups.
What is acetal in organic chemistry?
Acetal: A functional group in which carbon is attached by single bonds to two ether oxygen and two carbons (or hydrogens). A thioacetal has two sulfur atoms in place of an acetal's two oxygen atoms. This disaccharide contains both an acetal moiety (C-O-C-O-C) and a hemiacetal moiety (C-O-C-O-H).
Why dry HCl is used in acetal formation?
Dry HCl protonates oxygen of aldehyde or ketone. This positively charged oxygen makes the carbon of carbonyl group highly electron deficient. This will make a nucleophile (like methanol,ethanol,ethane diol,etc.) to attack on carbonyl carbon.
How do you identify acetal?
3:4611:46Acetal Ketal Hemiacetal Hemiketal Reaction Overview and ShortcutYouTubeStart of suggested clipEnd of suggested clipWhen you have a carbon that has a hydrogen and two o R groups attached. That's how you recognize anMoreWhen you have a carbon that has a hydrogen and two o R groups attached. That's how you recognize an acetone. If you have a carbon with two R groups attached.
What's the difference between acetal and ketal?
Review of acetals and ketals in general ? The term "acetal" used to be restricted to systems derived from aldehydes and the term "ketal" applied to those from ketones, but chemists now use acetal to describe both.
What is the difference between nylon and acetal?
One of the most obvious differences between nylon and acetal lies is aesthetics; acetal is shiny, while nylon appears dull in comparison. They also vary in regard to performance. For example: Compared to acetal, nylon offers superior tensile strength and bending stiffness.
What is the difference between acetal and Delrin?
Therefore, Delrin is a derivative of acetal. All Delrin is acetal but not all acetal is Delrin. Delrin is a type of acetal produced by the company named DuPoint. The main difference between acetal and Delrin is that acetal can be found as either a homopolymer or a copolymer whereas Delrin can be found as a homopolymer.
What is the difference between hemiacetal and acetal?
Hemiacetal is a byproduct of the acetal production process. Acetalization is the process of forming acetal. The primary distinction between acetals...
Why are acetals more stable than hemiacetals?
Cyclic acetals are more stable than regular acetals because of the chelate effect, which derives from having both -OH groups of the acetal connecte...
What is an acetal functional group?
A functional group in which carbon is attached by single bonds to two ether oxygen and two carbons (or hydrogens). A thioacetal has two sulfur atom...
What is the hemiacetal form?
A hemiacetal forms when an aldehyde reacts with an alcohol. There are two different ways this can occur, as a neutral reaction or catalyzed with an...
What is cyclic acetal?
A cyclic acetal is an acetal in the molecule of which the acetal carbon and one or both oxygen atoms thereon are members of a ring.
What happens to alcohol after protonation?from chem.libretexts.org
After protonation, an alcohol undergoes nucleophilic addition to the carbonyl group initially forming a hemiacetal upon deprotonation. Further protonation of the OH group in the hemiacetal allows for the elimination of water to form an oxonium ion. A second alcohol nucleophile adds to the oxonium ion to produce a protonated acetal. After deprotonation, the product acetal is formed.
Why are acetals more stable towards hydrolysis than acyclic ones?from chem.libretexts.org
Because both OH groups are part of the same molecule, the second nucleophilic addtion in the formation of the acetal is intramolecular and forms a ring. Cyclic acetals are more stable towards hydrolysis than acyclic ones and are also kinetically favored because the intramolecular ring-closing reaction is fast.
Why are acetals important?from chem.libretexts.org
The importance of acetals as carbonyl derivatives lies chiefly in their stability and lack of reactivity in neutral to strongly basic environments. As long as they are not treated by acids, especially aqueous acid, acetals exhibit all the lack of reactivity associated with ethers in general. Among the most useful and characteristic reactions of aldehydes and ketones is their reactivity toward strongly nucleophilic (and basic) metallo-hydride, alkyl and aryl reagents. If the carbonyl functional group is converted to an acetal these powerful reagents have no effect; thus, acetals are excellent protective groups, when these irreversible addition reactions must be prevented.
What happens when a carbonyl functional group is converted to an acetal?from chem.libretexts.org
If the carbonyl functional group is converted to an acetal these powerful reagents have no effect ; thus, acetals are excellent protective groups, when these irreversible addition reactions must be prevented. In the following example we would like a Grignard reagent to react with the ester and not the ketone.
Why is intramolecular hemiacetal formation common in carbohydrate chemistry?from chem.libretexts.org
Because sugars often contain alcohol and carbonyl functional groups, intramolecular hemiacetal formation is common in carbohydrate chemistry as we will see in Section 25.7. For example, the common sugar glucose exists in the cylcic manner more than 99% of the time in a mixture of aqueous solution.
What are the mechanisms of hemiacetal and acetal formation?from chem.libretexts.org
1) Protonation of the carbonyl. 2) Nucleophilic attack by the alcohol. 3) Deprotonation to form a hemiacetal. 4) Protonation of the alcohol. 5) Removal of water. 6) Nucleophilic attack by the alcohol.
What are thioacetals?from khanacademy.org
Thioacetals are similar to acetals, but form from reaction of an aldehyde with a thiol (not an alcohol). Created by Jay. Google Classroom Facebook Twitter. Email. Reactions of aldehydes and ketones. Formation of hydrates. Formation of hemiacetals and hemiketals. Acid and base catalyzed formation of hydrates and hemiacetals. Formation of acetals.
What is the difference between acetal and copolymer?from industrialspec.com
Acetal homopolymer may contain a lower-density or porous center. Porosity in a plastic means it may contain small bubbles or voids. These allow gases and liquids to seep into the plastic. Copolymer acetals have little or no porosity at their centers.
What is glass filled and glass reinforced?from industrialspec.com
Glass-filled and glass-reinforced acetal plastics. One other type of acetal plastic that is sometimes used to make flow control parts is glass filled or glass reinforced acetal. The glass used in glass filled and glass reinforced acetal plastics is actually chopped glass fibers.
How to contact dielectric manufacturing?from dielectricmfg.com
Fixtures. Contact a Dielectric Manufacturing knowledge expert to discuss the use of acetal for fabrication of your thermoplastic parts. Call 800-367-9122 or email [email protected].
How hot does semi crystalline plastic melt?from industrialspec.com
Typically, semi-crystalline thermoplastics melt and flow at temperatures ranging from about 275º to 335º F (135º to 335º C).
What is glass filled acetal?from industrialspec.com
For glass filled acetals, the glass fibers act as a filler and make the parts stiffer but not necessarily stronger. Fiber reinforced acetals use glass fibers that have been sized and chemically treated to help them stick to acetal plastic. Glass reinforcement provides both stiffness and strength. Glass filled acetals.
What is a push in swivel?from industrialspec.com
What is Acetal Plastic? Acetal plastic, also called polyacetal and polyoxymethylene (POM), is a general purpose, semi-crystalline, engineered thermoplastic. Acetal is commonly used for parts that need to be very stiff, have low surface friction and good dimensional stability. Dimensional stability is the ability ...
Why are thermoplastics used in injection molding?from industrialspec.com
Thermoplastics are useful for making products because they can be heated until they melt and flow. After cooling, thermoplastics become hard again. This cycle of melting, reforming and cooling can be repeated again and again. Injection molding is a production process that takes advantage of this.
How to Bond Acetal?from permabond.com
Permabond TA46XX Series structural acrylic adhesives form very strong water-resistant bonds to acetal.
What is the best replacement for sheaves?from plastichowto.wordpress.com
Nylon is fairly easy to find and cost-effective for most sheaves. Acetal: An easily machined and very hard plastic, acetal is known as a nylon replacement – especially in “wet” or marine applications. Sheaves for boating are a fairly common application – and in that situation acetal should be your choice.
What is the difference between acetal and UHMW?from plastichowto.wordpress.com
Two that seem similar to the uninformed are acetal and UHMW. However, as the YouTube video we found shows – machining the two materials is very different. Acetal is extremely hard and machines to close tolerances without “gumming” up lathes. UHMW on the other is soft and is difficult to machine and suffers from thermal expansion. Both materials are impervious to moisture and come in FDA compliant forms. Price is a major factor between the two as UHMW is much more economical and this means for projects that do not require maximum precision, UHMW will usually perform very well. An example “diy” UHMW application would be sled tracks, popular in Arctic locations.
How to stop a delrin from spinning?from cloudynights.com
Using a drill press I have gotten away with doing this a few times. Turn on the drill with the tap in it. Turn the drill off and lower it into the plastic before it stops spinning. It will seat straight into the delrin a few turns and stop, you then unchuck the tap and continue tapping the hole.
How well did tapping the moonlite connectors go?from cloudynights.com
Tapping the Moonlite connectors went extremely well. I first practiced on one of the holes I didn't intend on using and that went really smoothly. I ended up doing all the taps by hand and didn't have a single problem.
What is acetal used for?from plastichowto.wordpress.com
As such, acetal is often used as a direct replacement for cast nylon in “wet” applications. Acetal comes in two forms, the homopolymer (white) and copolymer (black). There used to be quality issues with the white but those have been remedied.
What is Delrin aceytal?from cloudynights.com
Delrin is also know as aceytal. If the hole is "blind" (not thru) you might want to use a spiral tap. The spiral tap will pull the chip through the top of the hole. They are not as robust as a plug tap, but it's worth it if you don't have to dig a chip out of the bottom of a hole.
What is the silyl ketal acetal variant?from sciencedirect.com
The silyl ketal acetal variant281 of the Claisen rearrangement of allyl alcohol ester enolates allows a predictable transfer of stereochemistry from starting material to product under very mild conditions. Ireland et al. have published full details 282 on the factors controlling the stereoselectivity in silyl ketene acetal formation, and on the preference 283 for a chair- or boatlike transition state in the actual [3,3]-sigmatropic shift.
What is the deprotection of the 5′-OH ketal group?from sciencedirect.com
It is important to prevent the loss of the 2 ′-OH protecting groups during the deprotection of the 5′-OH. New derivatives 1- (2-chloro-4-methylphenyl)-4-methoxypiperidin-4-yl ( 19) and 1- [ (2-fluoro) phenyl]-4-methoxypiperidin-4-yl (Fpmp, ( 20 )) were proposed as 2′-OH protecting groups by Reese and co-workers. 18,19 Under strong acidic conditions, the nitrogen atom of the piperidine ring of these protecting groups is protonated, which inhibits cleavage of the 2′- O -ketal function. At a lower proton activity (pH2), the nitrogen atom is largely unprotonated, and then the 2′- O -ketal function is cleaved easily. Since the dihydro derivative of ( 19) is difficult to prepare, ( 20) is frequently used to protect the 2′-OH. Scheme 4 shows the synthesis of 5′- O -Px-2′- O -Fpmp amidite units ( 26 ). The exocyclic amino-protected nucleoside is protected with a bifunctional reagent, 1,3-dichloro-1,1,3,3-tetraisopropyldisiloxane ( 27) (Markiewicz reagent), to give the 3′,5′-TBDMS-nucleoside ( 23 ). 20,21 The 2′-OH of ( 23) is protected to yield ( 20 ), and the 3′- and 5′-protecting groups are removed by fluoride anions to yield a 2- O -Fpmp nucleoside ( 24 ). After the 5′-OH of ( 24) is protected by Px-chloride, the 3′-OH of ( 25) is subjected to phosphitylation by 2-cyanoethyl- N, N -diisopropylchloro-phosphoramidite ( 28 ).
What is the formula for ketal?from chem.libretexts.org
A ketal is a compound that has the following general structural formula. R 3, R 4 = alkyl (In most ketals, R 3 = R 4; cyclic ketals are an exception.) The functional group 1 in an organic molecule is called the ketal group; the carbon atom bearing the two oxygen atoms is the ketal carbon.
How are glycosidic bonds formed?from chem.libretexts.org
Reactions in which new glycosidic bonds are formed are catalyzed by enzymes called glycosyltransferases, and in organic chemistry terms these reactions represent the conversion of a hemiacetal to an acetal (remember that sugar monomers in their cyclic form are hemiacetals and hemiketals). The mechanism for glycosidic bond formation in a living cell parallels the acid-catalyzed (non-biological) acetal-forming mechanism, with an important difference: rather than being protonated, the O H group of the hemiacetal is converted to a good leaving group by phosphorylation (this is a pattern that we are familiar with from chapters 9 and 10). The specific identity of the activating phosphate group varies for different reactions, so it is generalized in the figure below.
What is the exercise 10.4?from chem.libretexts.org
Exercise 10.4. 1. For each acetal / ketal A-D in the figure above, specify the required aldehyde / ketone and alcohol starting materials. Exercise 10.4. 2. Categorize each of the following molecules as a hemiacetal, hemiketal, acetal, ketal, hydrate of an aldehyde, or hydrate of a ketone. Exercise 10.4. 3.
What enzymes catalyze the cleavage of glycosidic bonds in carbohydrates?from chem.libretexts.org
The general mechanism above applies to reactions catalyzed by glycosidase enzymes , which catalyze the cleavage of glycosidic bonds in carbohydrates. In the introduction to this chapter, we learned about ongoing research in the field of cellulosic ethanol. Recall that the main bottleneck in the production of ethanol from sources such as switchgrass or wood is the cellulase-catalyzed step in which the glycosidic bonds in cellulose are cleaved. Cellulose-digesting microbes have several different but closely related forms of cellulase enzymes, all working in concert to cleave cellulose into smaller and smaller pieces until individual glucose molecules are free to be converted to ethanol by the fermentation process. Below is a representative mechanism for a cellulase reaction.
What is the carbon of the left side of a monomer?from chem.libretexts.org
If you look carefully, you should recognize that carbon #1, the anomeric carbon on the left-side glucose monomer, is the central carbon of an acetal group. Biochemists refer to this as a b-1,4 linkage, because the stereochemistry at carbon #1 is b in the specialized carbohydrate nomenclature system, and it is linked to carbon #4 ...