
How do you make 0.1 KMnO4?
- Dissolve 3.2 g of Potassium Permanganate (KMnO4) in 100 mL of water and dilute the solution with water to 1 L.
- Allow the solution to stand in the dark for two weeks and then filter through a fine-porosity sintered-glass crucible. Do not wash the filter.
Why can't we titrate KMnO4 solution with hydrochloric acid?
This titration cannot be carried out in the presence of acids like nitric acid or hydrochloric acid because itself is an oxidising agent. So hydrochloric acid chemically reacts with KMnO 4 solution forming chlorine which is also an oxidising agent.
How do you titrate MnO4 + 8H+ + 5fe2+ + 4H2O?
Overall ionic equation – MnO4– + 8H+ + 5Fe2+ → Mn2+ + 5Fe3+ + 4H2O This titration is based upon oxidation-reduction titrations. When ferrous ammonium sulfate solution is titrated against potassium permanganate in the presence of acidic medium by sulfuric acid. Acidic medium is necessary in order to prevent precipitation of manganese oxide.
What is the oxidising ability of KMnO4 in acidic medium?
In acidic medium the oxidising ability of KMnO 4 is represented by the following equation. Solution containing MnO 4– ions are purple in colour and the solution containing Mn 2+ ions are colourless and hence permanganate solution is decolourised when added to a solution of a reducing agent.
How do you find the strength of KMnO4?
The strength of and molarity of given KMnO 4 solution is found out as 2/y x 31.6 g/l and N/5 moles/liter, respectively. Potassium permanganate is dark, so always read the upper meniscus. Rinse the pipette and burette before use. Use dilute sulfuric acid for acidifying the potassium permanganate.
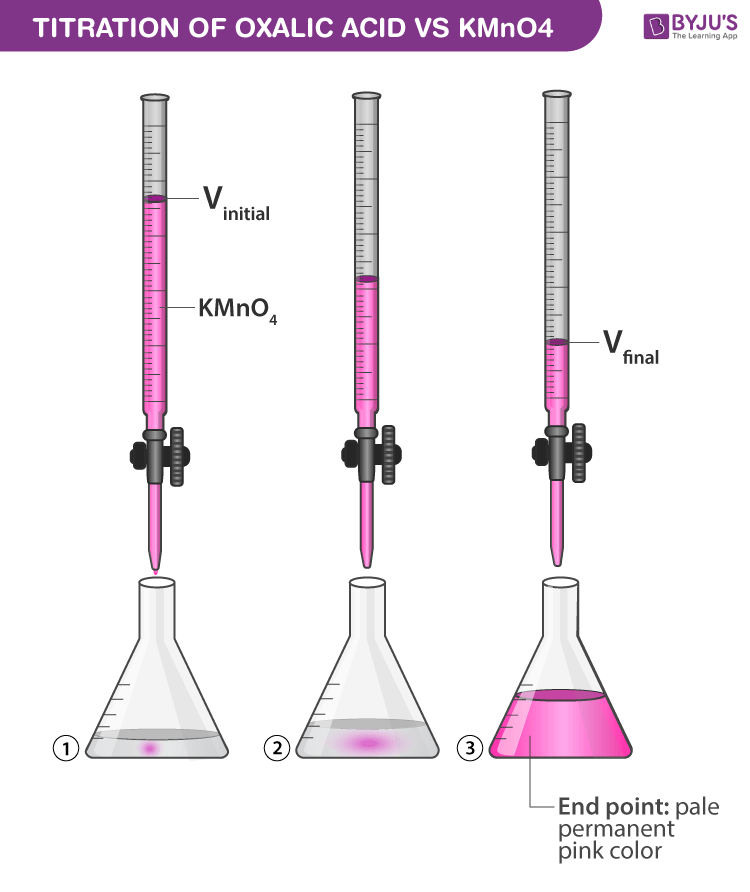
How do you prepare KMnO4?
Preparation Of Potassium Permanganate – KMnO4 Potassium permanganate is commercially prepared by mixing solution of KOH and powdered manganese oxide, with oxidizing agents like potassium chlorate. The mixture is boiled evaporated and the residue is heated in iron pans until it has acquired a pasty consistency.
How will you prepare 100ml 0.1 N KMnO4 solution?
0.1 N Potassium permanganate Solution Dissolve 3.2 g of Potassium Permanganate (KMnO4) in 100 mL of water and dilute the solution with water to 1 L. Allow the solution to stand in the dark for two weeks and then filter through a fine-porosity sintered-glass crucible.
How do you make one normal solution of KMnO4?
Molecular mass of KMnO4 = 39 + 55 + 16*4 = 158 Here volume and normality is 1 In order to prepare 1 normal solution (1 litre) of potassium permanganate N = (mass/Equivalent mass) * 1/(Volume of solution in litre) 1= mass/(158/5)*1/1 litre {as equivalent mass = molar mass/no.
How do you prepare KMnO4 solution for titration with oxalic acid?
Take 10 ml of oxalic acid solution in a clean conical flask. Add 5ml of 1.0M sulphuric acid to it. Heat the solution up to 50-60℃ before titrating it with potassium permanganate solution. To increase the visibility of the colour, keep the white tile below the conical flask.
How do you prepare 0.01 KMnO4?
Calculate the mass required to prepare a 250 mL 0.01 M solution of KMnO4? Mass = # moles x molar mass Molar mass of KMnO4 = 158.0 g/mole Mass of KMnO4 needed = 0.0025 mol x 158.0 g/mole = 0.395 g of KMnO4 So, weigh 0.395 g of KMnO4 and dissolve them in 250 ml volumetric flask.
How do you make 0.1 N from 1 N?
So take with a pipette 100 ml of the 1N solution and drop it in a 1000 ml volumetric flask, add water to the 1000 ml mark, shake it, done, there is your 1 liter 0.1 N solution.
Why is freshly prepared KMnO4 used in titration?
Potassium permanganate acts as a self-indicator in this titration. The appearance of the endpoint can be detected by the colour change of KMnO4 from colourless to light pink.
How is 5% solution of KMnO4 is prepared?
The mass of KMnO4 is 150 grams. Hence to make 5 % solution of KMnO4 you will need 7.5 g of KMnO4.
How will you prepare 0.1 normal solution?
1.99 g of NaOH must be diluted to 500 mL to prepare a 0.1N solution.
Why only h2so4 is used in KMnO4 titration?
Sulfuric Acid is generally used for this purpose because Nitric Acid and Hydrochloric Acid can participate in competing oxidation-reduction reactions, reducing the accuracy of the titration.
How do you prepare the acidified solution of KMnO4?
Dissolve 3.2 g of potassium permanganate in 1000 ml of water. Heat on a water-bath for 1 hour. Allow to stand for 2 days and filter through glass wool.
Which one is used for titration with KMnO4 in acidic medium?
dilute sulphuric acidTo determine the concentration/molarity of KMnO4 solution by titrating it against a 0.1 M standard solution of oxalic acid. The acid used in this titration is dilute sulphuric acid.
How would you prepare 100ml 0.1 N NaOH solution?
This is an Expert-Verified Answer To make 0.1N NaOH solution = dissolve 40 grams of NaOH in 1L of water. For 100 ml of water = (4/1000) × 100 = 0.4 g of NaOH. Thus, the amount of NaOH required to prepare 100ml of 0.1N NaOH solution is 0.4 g of NaOH.
How would you prepare 100ml 0.1 N HCL?
37 ml of solute/100 ml of solution. Therefore add 8.3 ml of 37% HCL to 1 liter of D5W or NS to create a 0.1N HCL solution.
How do you make a 0.1 N solution?
To prepare 0.1N NaOH solution we should dissolve 40 grams of NaOH in 1L of water and to standardize we should Use titration method.
How will you prepare 1000 ml of 0.1 M and 0.1 N sulfuric acid solution?
Sulphuric Acid Solution Standardization Dissolve it in 100 ml of water and add 0.1 ml of methyl red solution. Add the acid slowly from a burette, with constant stirring, until the solution becomes faintly pink. Heat the solution to boiling, cool and continue the titration.
Define titrant and titre.
The titre of a solution is the weight of a substance that reacts with 1 mL of the solution, such as a titrant.
Why is dil.sulfuric acid suitable for permanganate titration?
KMnO 4 acts as a good oxidising agent in acidic medium. If acid is not used KMnO 4 may be oxidised to MnO 2 giving a brown precipitate.
What is the formula for Mohr’s salt?
The formula for Mohr’s salt is (NH 4 ) 2 Fe(SO 4 ) 2 .6H 2 O.
What is the standard solution?
A standard solution is a solution whose concentration is known. The normality and molarity of the solution is known.
What are the different types of titration?
The different types of titration are: Iodometric titration Permanganate titration Complexometric titration Precipitation titration Acid-base titrat...
Table of Contents
Aim Theory Materials Required Apparatus Setup Procedure Observations Calculations Results and Discussion Precautions Viva Questions
Theory
Potassium permanganate is a strong oxidant in the presence of sulfuric acid. Mohr salt is a double salt forming a single crystalline structure having the formula (NH 4) 2. FeSO4. 6H2O. The chemical name for Mohr’s salt is ferrous ammonium sulfate.
Calculations
Consider y ml of given KMnO 4 solution are equivalent to 20ml of N/10 Mohr’s salt solution.
Viva Questions
The titre of a solution is the weight of a substance that reacts with 1 mL of the solution, such as a titrant.
