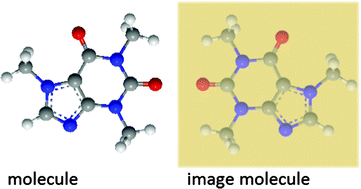
How to make a mirror image for a molecule?
May 07, 2020 · How to Draw People in Mirrors Draw the person the mirror is going to reflect first. Draw a straight line from the top of the person's head to the mirror. Decide which part of the head will be reflected in the mirror. Draw the reflection smaller if the mirror is far away from the person. Erase your guideline you drew in step two.
What are mirror-image stereoisomers?
Dec 28, 2015 · When checking out chirality or achirality you are always allowed and supposed to rotate the molecule freely in all dimensions. Essentially, what you are trying to prove, is that the mirror image of a molecule can be achieved by rotation alone. The thought behind this is simple. Chirality is macroscopically proven by optical activity.
Are mirror images of chiral and Achiral molecules superposable?
There is no chemical test you could perform to differentiate them. This is true for all achiral compounds. The most interesting case of this would be a class of molecules called meso compounds. They contain multiple stereogenic centers, but due to an internal plane of symmetry (or certain other symmetry elements ), reflection of these molecules across a mirror plane …
What properties make enantiomers unique to their mirror images?
How do you draw a mirror image of an organic compound?
0:462:53Drawing Enantiomers Fast Hacks - YouTubeYouTubeStart of suggested clipEnd of suggested clipThere are two ways two ways the first way as we know from a name in antemer means a mirror image. SoMoreThere are two ways two ways the first way as we know from a name in antemer means a mirror image. So you could imagine that's an invisible mirror here and you draw the molecule in the opposite way.
How can you tell if two molecules are mirror images?
When deciding if two groups are stereoisomers or the same, you need to answer the question whether the two molecules are mirror images. One way to do this is to draw the mirror image of the first molecule and see if it is the same as the second.
What is the mirror image of a molecule?
Enantiomers — Configurational isomers that are related as mirror images. Enantiomers must be chiral; and every chiral molecule has one, and only one, enantiomer. Note that in practical terms, Enantiomers means: "two molecules which are mirror images of each other but which can't be superimposed without breaking bonds".
How do you create a mirror image?
1:0010:08Cycle 3 Week 2 drawing mirror images - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd making it reflect from that center line so we use the mirror to check air to see what thatMoreAnd making it reflect from that center line so we use the mirror to check air to see what that reflection should look like. So if we put the mirror on that line of symmetry.
How do you find a mirror image?
0:219:34Determining Chirality by Generating the Mirror Image - YouTubeYouTubeStart of suggested clipEnd of suggested clipIf we find that the mirror image is not the same in other words we cannot perfectly overlay the twoMoreIf we find that the mirror image is not the same in other words we cannot perfectly overlay the two structures with no differences. Then we know immediately that the original molecule is chiral.
What is a mirror image isomer?
One of the most interesting types of isomer is mirror-image stereoisomers, a non-superimposable set of two molecules that are mirror images of one another. The existence of these molecules is determined by concept known as chirality.
What is a superimposable mirror image?
Superimposable Many objects (including molecules) are indistinguishable from their mirror images, so they are superimposable. Non-superimposable Other objects, such as your left and right hands, can be distinguished, they are non-superimposable.
How do you find a superimposable mirror image?
The most straightforward way to determine whether a given object is chiral is to draw or visualize the object's mirror image and see if the two are identical (that is, superimposable). If the object contains an internal plane of symmetry then it must be achiral.
How do you draw a mirror reflection?
0:261:43How to draw a Mirror Real Easy - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd that will be a little bit of kind of shading kind of stuff down there and then that would beMoreAnd that will be a little bit of kind of shading kind of stuff down there and then that would be thick around that side. And then what you want is to is all at the same angle.
How do you draw a mirror in science?
2:458:00How To Draw Ray Diagrams | Reflection Of Light| Physics | ScienceYouTubeStart of suggested clipEnd of suggested clipThe image appears to come from a point behind the mirror. Let's call this point a - the point a -MoreThe image appears to come from a point behind the mirror. Let's call this point a - the point a - can only be formed once the reflected rays PP. - and QQ - are produced backwards.
What gives the mirror image of a drawing?
Answer: Go to the Format tab and find the Rotate option. Then select "Flip Vertical" and "Flip Horizontal" option to mirror the image.Feb 26, 2021
How to make a dichloromethane molecule?
To prepare a dichloromethane molecule, get a carbon atom (black) and connect two Hydrogen atoms (white) using short connectors. 2. To the two remaining holes, attach two chlorine atoms (green) using medium connectors. 3.
How to tell if a compound is chiral or not?
To know if the compound is chiral or not, rotate the molecule in any way and see if the molecules will look exactly like each other. For the molecule given. Rotate the compound by 180° by holding the Hydrogen atom on top. 5.
What is a chiral compound?
Chiral compounds are compounds whose molecules are non-superimposable to their mirror image. If a compound is superimposable to its mirror image, it is classified as an achiral compound. An example of an achiral compound is methane.
Why is chirality important?
The concept of chirality is important as it tells us about the ability of molecules to rotate plane-polarized light. Depending on the chirality of the molecule, it can rotate the light either to the left or to the right.
What is stereochemistry?
Stereochemistry is a sub-discipline of chemistry that involves the study of the relative spatial arrangements of atoms within a molecule. It also covers studying the effect of the spatial arrangements on the physical and chemical properties of compounds. One of the main foci of stereochemistry is determining the chirality of molecules.
What is the name of the liquid that dissolves organic compounds?
Additional Information: Dichloromethane, also called methylene chloride, is a clear volatile liquid that has the formula CH2Cl2. It is volatile and has the ability to dissolve a number of organic compounds. It is also used as paint stripper and degreaser. 1. 1.
Is chloromethanol an amino group?
2. Now, prepare the structure of 1-amino-1-chloroethanol by replacing one of the hydrogen atoms of chloromethanol with an amino group (N atom connected to two H atoms using small connectors).
Where is the mirror image of A?
The mirror image of A, which we will call B, is drawn on the right side of the figure, and an imaginary mirror is in the middle. Notice that every point on A lines up through the mirror with the same point on B: in other words, if A looked in the mirror, it would see B looking back.
How to tell if a molecule is chiral?
The easy way to determine if a molecule is chiral is simply to look for the presence of one or more chiral centers: molecules with chiral centers will (almost always) be chiral.
What is the opposite of chiral?
The opposite of chiral is achiral. Achiral objects are superimposable with their mirror images. For example, two pieces of paper are achiral. In contrast, chiral molecules, like our hands, are non superimposable mirror images of each other.
What are the two types of stereoisomers?
There are two types of stereoisomers: enantiomers and diastereomers. Enantiomers are pairs of stereoisomers which are mirror images of each other: thus, A and B are enantiomers. It should be self-evident that a chiral molecule will always have one (and only one) enantiomer: enantiomers come in pairs.
What is a chiral hand?
The term chiral, from the Greek work for ‘hand’, refers to anything which cannot be superimposed on its own mirror image. Your hands, of course, are chiral – you cannot superimpose your left hand on your right, and you cannot fit your left hand into a right-handed glove (which is also a chiral object).
What is the fundamental of chirality?
Fundamentals of Chirality. Stereoisomers are isomers that differ in spatial arrangement of atoms, rather than order of atomic connectivity. One of their most interesting type of isomer is the mirror-image stereoisomers, a non-superimposable set of two molecules that are mirror image of one another. The existance of these molecules are determined by ...
Why does the face have symmetry?
Your face has a plane of symmetry, because the left side is the mirror image of the right side. What Pasteur, Biot, and their contemporaries did not yet fully understand when Pasteur made his discovery of molecular chirality was the source of chirality at the molecular level.
What are the two stereochemical relationships between two molecules?
Enantiomers and diastereomers are the only two stereochemical relationships that you can have between any two molecules. The stereoisomers are any two molecules that fulfill the following two requirements: Both molecules must have the same atom connectivity.
How to make your thumbs look different?
Think about your hands. If you align them together so that all fingers line up, the palms are going to be looking in the opposite directions. If you make your palms look in the same direction, your thumbs will be looking in different direction, etc.
What is diastereomer in science?
The official definition though is the diastereomers are non-superimposable molecules that are not mirror images of each other. For instance, let’s look at the following two molecules: Molecules (3) and (4) are obviously not mirror images, so they cannot be enantiomers. They are also not superimposable in space no matter how much you rotate those in ...
Can you build an allene with a molecular model kit?
If you build a pair of allenes with your molecular model kit (yes, get your molecular model kit and actually build those!), you’ll see, that those are not superimposable in space. But these two molecules have no chiral carbons… and yet, they fit the definition of enantiomers, therefore they are a pair of enantiomers!
Do molecule and molecule have the same group?
While molecule (1) and molecule (2) have both groups (OH and Br) cis to each other, they look in different directions from the plane of the cycle. Note, there are many ways of how you can make a mirror image for a molecule. Here are the three possible examples:
Do stereochemical relationships focus on chiral atoms?
Many students tend to have a sort of a tunnel vision when it comes to stereochemical relationships focusing only on molecules with chiral atoms. This is a faulty heuristic! So, make sure you always analyze the entire molecule and use the definition of the relationship, rather than only focusing on the chiral atoms.
