How do you name amides and amines?
The prefix form is both “carbamoyl-” and “amido-“. Amides that have additional substituents on the nitrogen are treated similarly to the case of amines: they are ordered alphabetically with the location prefix N: HCON(CH3)2 is N,N-dimethylmethanamide.
How do you name and draw an amide?
8:2710:14The parent name also always includes in that fork in that chain of carbons the carbonyl carbon andMoreThe parent name also always includes in that fork in that chain of carbons the carbonyl carbon and the carbonyl carbon always gets carbon number one there's carbon two carbon three.
How do you name amines?
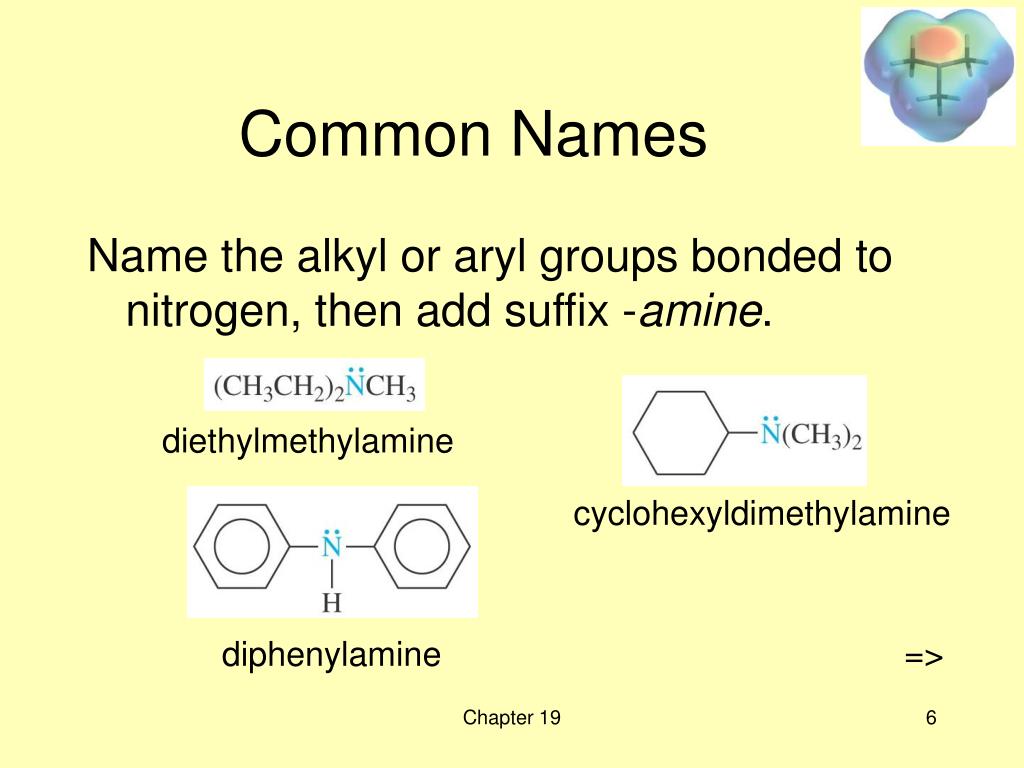
Simple 1°, 2°, and 3° amines: common (trivial) names are obtained by alphabetically arranging the names of the alkyl substituents on the nitrogen and adding the suffix -amine (e.g., ethylmethylamine). Amines in the IUPAC system: the “e” ending of the alkane name for the longest chain is replaced with –amine.
How do you name secondary and tertiary amides?
814.1 - Symmetrical secondary and tertiary amines are named by adding to the name of the radical a prefix "di-" or "tri-", respectively, and the suffix "-amine".
What is the group name for an amide?
The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine.
Is NH2 amine or amide?
More complex primary amines are named with —NH2 as the amino substituent.
What amide mean?
Definition of amide 1 : an inorganic compound derived from ammonia by replacement of an atom of hydrogen with another element (such as a metal) 2 : any of a class of organic compounds derived from ammonia or an amine by replacement of hydrogen with an acyl group — compare amine, imide.
What is difference between amine and amide?
Amine is an ammonia derivative in which one or more hydrogen atoms are replaced by an alkyl or aryl group, while Amide is an amine derivative of carboxylic acid. A sigma bond joins a carbonyl carbon atom to a nitrogen atom bonded by hydrogen atoms or carbon atoms.
How do you name amines A level chemistry?
2:133:07Them we name amines using the same technique as you've seen to name lots of other organic molecules.MoreThem we name amines using the same technique as you've seen to name lots of other organic molecules. We base the name of the root of it on the longest alkyl chain attached to the nitrogen.
How do you name a tertiary amide?
3:2011:25So in this example we have a tertiary amide the nitrogen atom is attached to three carbon atoms soMoreSo in this example we have a tertiary amide the nitrogen atom is attached to three carbon atoms so go ahead and name this amide. So let's count the longest chain. It's a six carbon chain.
How do you identify amide?
Amides have a general structure in which a nitrogen atom is bonded to a carbonyl carbon atom. In names for amides, the -ic acid of the common name or the -oic ending of the IUPAC for the corresponding carboxylic acid is replaced by -amide.
How do you read amide?
As with amines, the nomenclature used for an amide depends on the number of carbons attached to the nitrogen. A primary (1°) amide has nitrogen attached to a single carbon; a secondary (2°) amide has the nitrogen attached to two carbons; a tertiary (3°) amide has the nitrogen attached to three carbons.
What is the structure of amide?
Amides have a general structure in which a nitrogen atom is bonded to a carbonyl carbon atom. In names for amides, the -ic acid of the common name or the -oic ending of the IUPAC for the corresponding carboxylic acid is replaced by -amide.
What is an example of an amide?
Nylon, paracetamol, and dimethylformamide are examples of amides. The most basic amides are ammonia derivatives. Amphetamines, in general, are very weak bases.
What is the general formula of amide?
Amide groups have the general chemical formula CO-NH.
How do you identify an amide functional group?
The amide functional group has an nitrogen atom attached to a carbonyl carbon atom. If the two remaining bonds on the nitrogen atom are attached to hydrogen atoms, the compound is a simple amide.
What is the suffix for amide?
Now, for primary amides, all you need to do is replace the -ic acid , or -oic acid ending with the suffix “ amide”.
Is amide carbon counted as a parent chain?
Notice that the amide carbon, in this case, is not counted as part of the parent chain.