Certain rules apply to the way names of covalent compounds are written:
- The more electropositive element (further left on the periodic table) is listed before the more electronegative element (further right on the periodic table).
- The second element is given an -ide ending.
- Prefixes are used to denote how many atoms of each element are present in the compound.
- Name the non-metal furthest to the left on the periodic table by its elemental name.
- Name the other non-metal by its elemental name and an -ide ending.
- Use the prefixes mono-, di-, tri-.... to indicate the number of that element in the molecule.
What are the rules for naming covalent compounds?
Naming Covalent Compounds • When naming Covalent Compounds follow these IUPAC rules: 1. The non-metal closest to the left of the periodic table goes first and keeps its name 2. The second non-metal element is named with the suffix “-ide” 3. “Descriptive Prefixes” are added to the beginnings of the names of both elements. These prefixes are
What are 5 examples of covalent bonds?
What are 5 examples of covalent bonds? Hydrogen (H 2) Hydrogen (H) is the simplest of all elements. … Oxygen (O 2) The valency of oxygen (O) is two, which means that it requires two electrons to complete its outermost (valence) shell. … Nitrogen (N 2) … Water (H 2 O) … Carbon Dioxide (CO 2) … Methane (CH 4) … Ammonia (NH 3) …
What are some examples of covalent compounds and their uses?
What are 4 compounds from your everyday life that are covalent?
- Water.
- Sugar.
- Oxygen.
- Carbon Dioxide.
- LPG.
- Vinegar.
- Nail Polish Remover.
- Diamonds.
When naming covalent compounds what does the prefix tell you?
When a pair of elements form more than one type of covalent compound, Greek prefixes are used to indicate how many of each element are in a compound. For example: Some of the Greek prefixes are given in the table below: The prefix mono is never used for naming the first element of a compound.
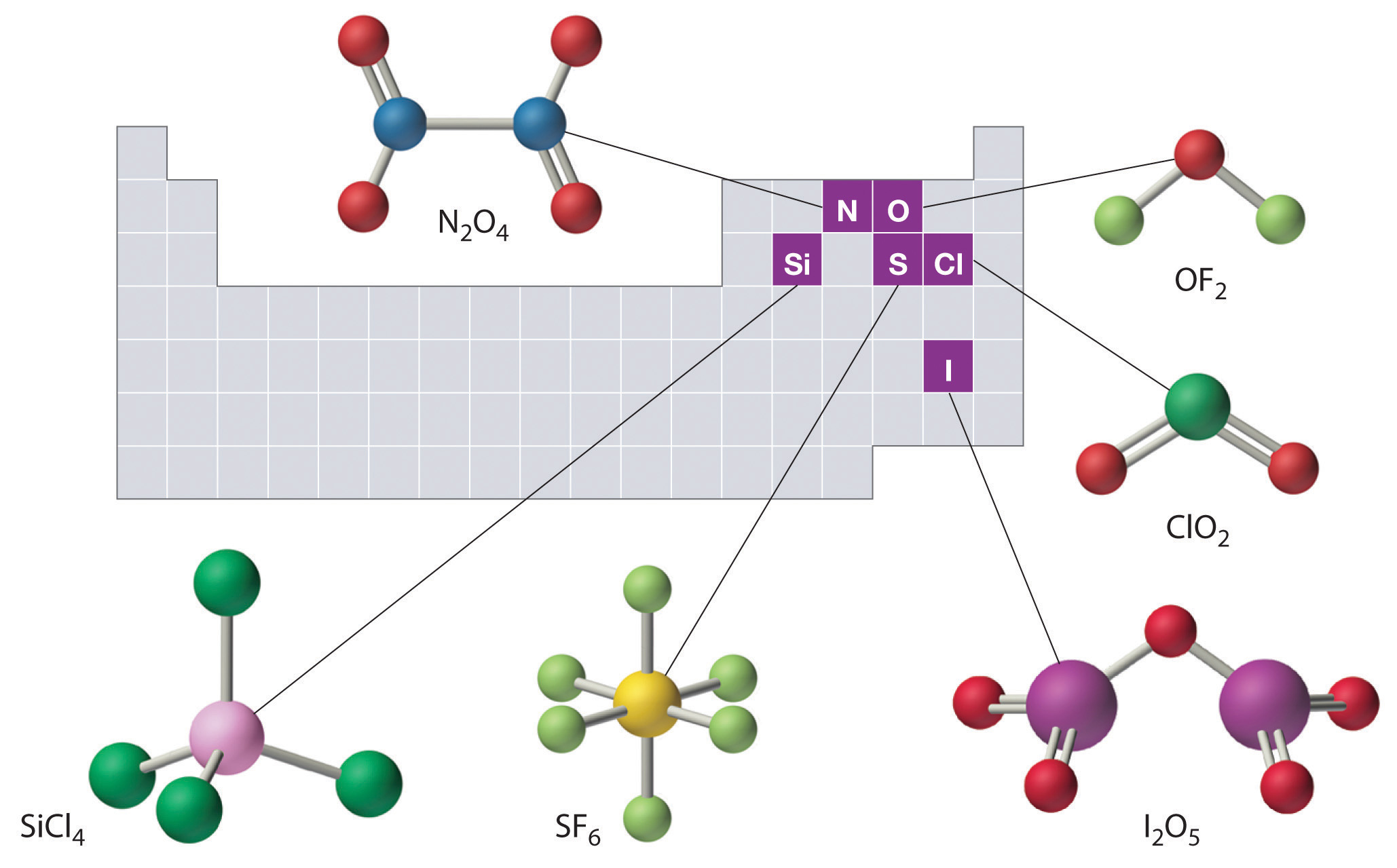
How do you name molecular compounds?
Molecular compounds are named with the first element first and then the second element by using the stem of the element name plus the suffix -ide. Numerical prefixes are used to specify the number of atoms in a molecule.
How do you name covalent and ionic compounds?
0:317:13Ionic and Covalent Compounds: Writing Names and FormulasYouTubeStart of suggested clipEnd of suggested clipIf it's covalent is going to be made from or true bond to non metal elements bonded together andMoreIf it's covalent is going to be made from or true bond to non metal elements bonded together and therefore you're going to use the numerical prefixes.
How do you identify a covalent compound?
2:014:17How to identify ionic compounds and covalent compounds? - Dr KYouTubeStart of suggested clipEnd of suggested clipWe can answer a few questions to easily classify the type of compound. If the compound containsMoreWe can answer a few questions to easily classify the type of compound. If the compound contains metal or ammonium ion like NH 4 plus then is an ionic compound otherwise is a covalent compound.
What is the rule for naming compounds?
A molecular compound is usually composed of two or more nonmetal elements. Molecular compounds are named with the first element first and then the second element by using the stem of the element name plus the suffix -ide. Numerical prefixes are used to specify the number of atoms in a molecule.
How do you name ionic compounds?
Ionic compounds are named by stating the cation first, followed by the anion. Positive and negative charges must balance. Some anions have multiple forms and are named accordingly with the use of roman numerals in parentheses.
How do you classify ionic and covalent bonds?
Compounds containing two elements (so called binary compounds) can either have ionic or covalent bonding. If a compound is made from a metal and a non-metal, its bonding will be ionic. If a compound is made from two non-metals, its bonding will be covalent.
How do you name ionic compounds step by step?
Rules for naming simple ionic compounds.Name the metal by its elemental name.Name the nonmetal by its elemental name and an -ide ending.Name metals that can have different oxidation states using roman numerals to indicate positive charge. Example Fe2+ is Iron(II) ... Name polyatomic ions by their names.
How do you tell if a compound is ionic or covalent by its formula?
NH3, H2O, etc. NaCl, MgO, etc. Overall, a bond is covalent if atoms have an electronegativity difference of less than 1.7. Conversely, if the electronegativity difference lies between 1.7 to 2.0 and metal is involved then the bond is considered ionic.
How to identify a molecular compound?
Identifying Molecular Compounds. Molecular compounds contain two or more nonmetals (not the ammonium ion). Usually, you can recognize a molecular compound because the first element in the compound name is a nonmetal. Some molecular compounds contain hydrogen, however, if you see a compound which starts with "H", ...
What is the nomenclature for covalent compounds?
Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. She has taught science courses at the high school, college, and graduate levels. Molecular compounds or covalent compounds are those in which the elements share electrons via covalent bonds.
How to write a formula for a covalent compound?
You can write the formula for a covalent compound from its name by writing the symbols for the first and second elements and translating the prefixes into subscripts. For example, xenon hexafluoride would be written XF 6. It is common for students to have trouble writing formulae from compounds names as ionic compounds and covalent compounds are often confused. You aren't balancing charges of covalent compounds; if the compound does not contain a metal, don't try to balance this!
What is a covalent compound?
Molecular compounds or covalent compounds are those in which the elements share electrons via covalent bonds. The only type of molecular compound a chemistry student is expected to be able to name is a binary covalent compound. This is a covalent compound made up of only two different elements.
Why do we use prefixes in compound?
Prefixes are used to denote how many atoms of each element are present in the compound.
Which element is listed before the more electronegative element?
Certain rules apply to the way names of covalent compounds are written: The more electropositive element (further left on the periodic table) is listed before the more electronegative element (further right on the periodic table). The second element is given an -ide ending.
Can you balance covalent and ionic compounds?
It is common for students to have trouble writing formulae from compounds names as ionic compounds and covalent compounds are often confused. You aren't balancing charges of covalent compounds; if the compound does not contain a metal, don't try to balance this!
What are covalent compounds?
A covalent compound, aka molecular compound, is a compound where two non-metals, or, a non-metal and a metalloid, are bonded together through the sharing of electrons.
Why do we omit the prefix "mono"?
If there is only one atom of the first element, omit adding a prefix because we will just assume there is only one. Also, the prefix mono is usually paired with oxygen (dihydrogen mono xide). Ultimately, we would end up with diboron hexahydride. Notice the last element has its root attached to “ide”.
What is a binary compound?
Binary compound: Compound with two different elements. Diatomic compound: Compound that has two atoms that can either be different elements or the same element. Covalent compound: Molecules formed by covalent bonds in which electrons are shared between atoms.
What is a compound that has two atoms that can either be different elements or the same element?
Diatomic compound: Compound that has two atoms that can either be different elements or the same element.
Which would go first, hydrogen or HCl?
For example, if we had HCl, hydrogen would go first because it is more electropositive than chlorine.
Which element would go first in a molecular formula?
If the molecular formula contains oxygen or a halogen (F, Cl, Br, I, At, Ts), then those elements will be placed first. For example, if we had HCl, hydrogen would go first because it is more electropositive than chlorine. 2. Prefixes and suffixes. Let’s say we have B 2 H 6.
Do you capitalize names in a letter?
Note: you don’t have to capitalize the names.
What are Covalent Compounds?
Covalent compounds are formed when two nonmetal atoms share valence electrons to form a covalent bond. Valence electrons are the atom’s outermost electrons. Elements want to fill their electron orbitals, or shells, with electrons, so they will form bonds with other atoms that will allow them to do so.
Rules for Naming Covalent Compounds
A binary compound is made up of only two elements. The names are referred to as systematic names.
Prefixes used for Covalent Compounds
Greek prefixes are used to name compounds based on the elemental subscript, which specifies the number of atoms present in the compound.
Naming Covalent Compounds With Three Elements
These are the rules for naming covalent compounds with three elements.
What is a covalent compound?
Naming Covalent Compounds (or "Molecular Compounds") When Given the Formula. Covalent compounds, often called "molecular compounds," consist of two non-metals; a non-metal / non-metal pair. These elements will be found on the far right side of the periodic table - to the right of the metalloid staircase. The simplest form of a covalent compound ...
What are ionic and covalent compounds made of?
Ionic compounds are made up of a metal cation (named first) and a non-metal anion (named second).
What is the difference between binary acids and oxyacids?
Binary acids are composed of hydrogen and one other element, while oxyacids contain hydrogen, oxygen, and another element. Translating binary acids from formula to name will end up in the format hydro[root of anion]ic acid.
What suffix is used to name ionic compounds?
In earlier years, chemists named ionic compounds containing metals with multiple charges using the suffixes -ic and -ous. This is not current practice in most areas of the scientific community and we will not discuss it here.
How to translate oxyacids from formula to name?
Translating oxyacids from formula to name involves dropping the word hydrogen completely, replacing the anion’s suffix, and adding acid. The oxyacid suffix replacements are -ate becomes -ic and -ite becomes -ous. Note that the definition of acid includes dissolved in water.
Can ionic compounds contain polyatomic ions?
Well it's also possible to have ion ic compounds that contain polyatomic ions. These are usually negatively charged "clumps" or "chunks" of ions that form bonds to the oppositely charged ion (usually a cation).
Is the process of covalent compounds simpler?
For covalent compounds the process is simpler , as the ratio of elements is indicated by the numerical prefixes.
What is the name of the acid that forms when HCl is dissolved in H2O?
For example, when gaseous HCl is dissolved in H2O, it forms hydro chlor ic acid. HCN in H2O is hydro cyan ic acid. Before we learn the rule for naming oxyacids, let's learn the rules for naming oxyanions.
What are some examples of covalent compounds?
Naming Binary Covalent Compounds. When a pair of elements form more than one type of covalent compound, Greek prefixes are used to indicate how many of each element are in a compound. For example: Compound. Name. N 2 O. dinitrogen monoxide. NO. nitrogen monoxide.
Is ClO4 an acid or an anion?
ClO- is hypo chlor ite. ClO2- is chlor ite. ClO3- is chlor ate. ClO4- is per chlor ate. Finally, here are the rules for naming acids of oxyanions . If the anion name ends in -ate, then the acid name ends in -ic or -ric. If the anion name ends in -ite, then the acid name ends in -ous .
When is the final o or a dropped?
The final o or a of a prefix is often dropped when the element begins with a vowel.
Is H2O always called water?
Finally, H2O, which according to the rules should be called dihydrogen monoxide is always called water, and NH3, or nitrogen trihydride, is always called ammonia.
Rules for naming molecular compounds
Molecular compounds are basically covalent compounds. They are mainly composed of nonmetals. For example, sulfur hexafluoride (SF 6) and dinitrogen pentoxide (N 2 O 5 ).
How to name simple molecular compounds?
There are several basic and common molecular compounds, such as hydrogen chloride (HCl). Being simple, they just have one name. There is no need to attach any prefixes or suffixes in order to indicate the number of molecules.
How to name complex molecular compounds?
Complex molecular compounds are composed of two or more different elements linked together. While naming them, it is a common practice in chemistry not to include symbols for each individual element. But rather use a single word (in italics) that reflects an overall category or class of elements.
CAS Number: A Unique Chemical Identifier
Chemical Abstract Service registry number of simply CAS number is a unique numerical identifier that can give particular information about a certain chemical compound. It serves as a unique code for a particular compound just like a person’s social security number or a car’s license plate number.
What are some examples of molecular compounds that don't dissolve well in water?
Examples of molecular compounds that don't dissolve well in water are oil and polymerized plastic. Note that network solids are compounds containing covalent bonds that violate some of these "rules". Diamond, for example, consists of carbon atoms held together by covalent bonds in a crystalline structure.
What are the properties of covalent bonds?
Properties of Covalent Compounds. Most covalent compounds have relatively low melting points and boiling points. While the ions in an ionic compound are strongly attracted to each other, covalent bonds create molecules that can separate from each other when a lower amount of energy is added to them. Therefore, molecular compounds usually have low ...
How much heat does it take to vaporize a mole of a liquid?
The enthalpy of vaporization is the amount of energy, at constant pressure, required to vaporize one mole of a liquid. On average, it takes only 1% to 10% as much heat to change the phase of a molecular compound as it does for an ionic compound. Covalent compounds tend to be soft and relatively flexible. This is largely because covalent bonds are ...
Why are covalent bonds so soft?
Covalent compounds tend to be soft and relatively flexible. This is largely because covalent bonds are relatively flexible and easy to break. The covalent bonds in molecular compounds cause these compounds to take form as gasses, liquids, and soft solids. As with many properties, there are exceptions, primarily when molecular compounds assume ...
Which compounds have lower melting and boiling points?
Therefore, molecular compounds usually have low melting and boiling points. Covalent compounds usually have lower enthalpies of fusion and vaporization than ionic compounds. The enthalpy of fusion is the amount of energy needed, at constant pressure, to melt one mole of a solid substance. The enthalpy of vaporization is the amount of energy, ...
What are the two atoms that make up flammable substances?
Many flammable substances contain hydrogen and carbon atoms which can undergo combustion, a reaction that releases energy when the compound reacts with oxygen to produce carbon dioxide and water. Carbon and hydrogen have comparable electronegativies so they are found together in many molecular compounds.
Do covalent compounds dissolve well in water?
Many covalent compounds don't dissolve well in water. There are many exceptions to this rule, just as there are many salts (ionic compounds) that don't dissolve well in water. However, many covalent compounds are polar molecules that do dissolve well in a polar solvent, such as water. Examples of molecular compounds that dissolve well in water are ...
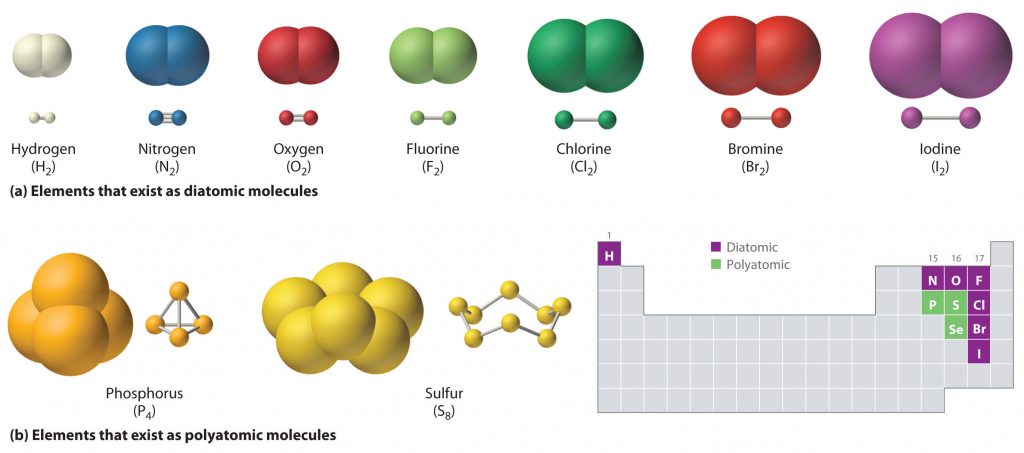
CORE Concepts
Topics Covered in Other Articles
Vocabulary
- Binary compound: Compound with two different elements.
- Diatomic compound: Compound that has two atoms that can either be different elements or the same element.
- Covalent compound: Molecules formed by covalent bonds in which electrons are shared between atoms.
What Are Covalent compounds?
- A covalent compound, aka molecular compound, is a compound where two non-metals, or, a non-metal and a metalloid, are bonded together through the sharing of electrons.
Examples
- What is the name of the diatomic compound Li2?
- What is the name of the binary compound NO?
- What is the name for the covalent compound HCl?
What Are Covalent compounds?
Table of Contents
Rules For Naming Covalent Compounds
Prefixes Used For Covalent Compounds
- Greek prefixes are used to name compounds based on the elemental subscript, which specifies the number of atoms present in the compound. Examples- 1. PCl5– Phosphorus pentachloride 2. SO2– Sulphur dioxide 3. CO2– Carbon Dioxide 4. N2O5– Dinitrogen pentoxide 5. BrF5– Bromine pentafluoride
Naming Covalent Compounds with Three Elements
Solved Examples