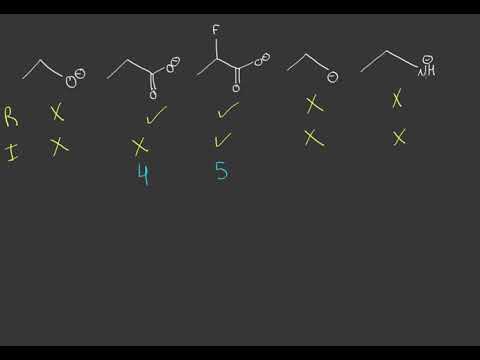
How do you determine which compound is most basic?
First, scan the molecule for all non-halogen atoms with lone pairs (usually N and O). Second, imagine protonating each candidate atom and draw its conjugate acid. Third, identify the weakest conjugate acid. The protonated atom in the weakest conjugate acid is the most basic atom in the original molecule.
How do you find the order of basicity?
The order of basicity is given as I > III > II > IV.
What does higher basicity mean?
In chemistry, the quality of being a base (not an acid). A base is a substance that can accept hydrogen ions in water and can neutralize an acid. Basicity is measured on a scale called the pH scale. On this scale, a pH value of 7 is neutral, and a pH value of more than 7 to 14 shows increasing basicity.
How do you rank acidity and basicity in organic chemistry?
11:3030:05Organic Chemistry - Ranking Acidity - YouTubeYouTubeStart of suggested clipEnd of suggested clipGuess charge stability phenomena here or if you have that negative charge the compound that is mostMoreGuess charge stability phenomena here or if you have that negative charge the compound that is most easily able to bear that negative charge is going to have the strongest conjugate acid.
Which among the following is correct order of basicity?
NH2- > OH- > RO- > RCOO.
Which of the following is strongest base?
In compound D, due to the presence of methylene group between amino group and the benzene ring, the resonance between the lone pair of electrons on N and the benzene ring is not possible. Hence, this lone pair can be readily donated, and hence, benzylamine is the strongest base among the given bases.
Does basicity increase down the group?
In general, basicity increases down a group (e.g., in the alkaline earth oxides, BeO < MgO < CaO < SrO < BaO). Acidity increases with increasing oxidation number of the element.
What makes a compound a good base?
The less electronegative an atom (the later it appears in the periodic table), the more basic it likely is. If electron density can be delocalized by resonance, the molecule is a weaker base as it is less interested in losing electrons and accepting a proton.
Why does basicity decrease down a group?
The basicity decreases with the size of the central atom due to diffusion of electrons over large volume i.e. down the group, as the size of the elements increases the electron density on element decreases.
How do you order bases from strongest to weakest?
2:013:02Ordering Acids and Bases - YouTubeYouTubeStart of suggested clipEnd of suggested clipYou basically flip the order. So the strongest acid we have here would be the weakest base we haveMoreYou basically flip the order. So the strongest acid we have here would be the weakest base we have here.
How do you compare basicity of organic compounds?
The less electronegative the element, the less stable the lone pair will be and therefore the higher will be its basicity. Another useful trend is that basicity decreases as you go down a column of the periodic table. This is because the valence orbitals increase in size as one descends a column of the periodic table.
Why does basicity increase up a group?
The ability of an atom “to accept an electron” decreases down the group therefore increases the tendency of the elements to be good Lewis bases (recall a Lewis base is an electron-pair donor).
What is the order of basicity of amines?
NH3< primary amine ~ tertiary amine < secondary amine.
How do you find acidity order?
In the following compounds, The order of acidity isA. III > IV > I > II.B. I > IV > III > II.C. II > I > II > IV.D. IV > III > I > II.
What is the order of basicity of P Methylaniline?
II > I > IV >III.
Which is correct order of basicity I Br Cl F?
Therefore the order of basicity is F>Cl>Br>I.