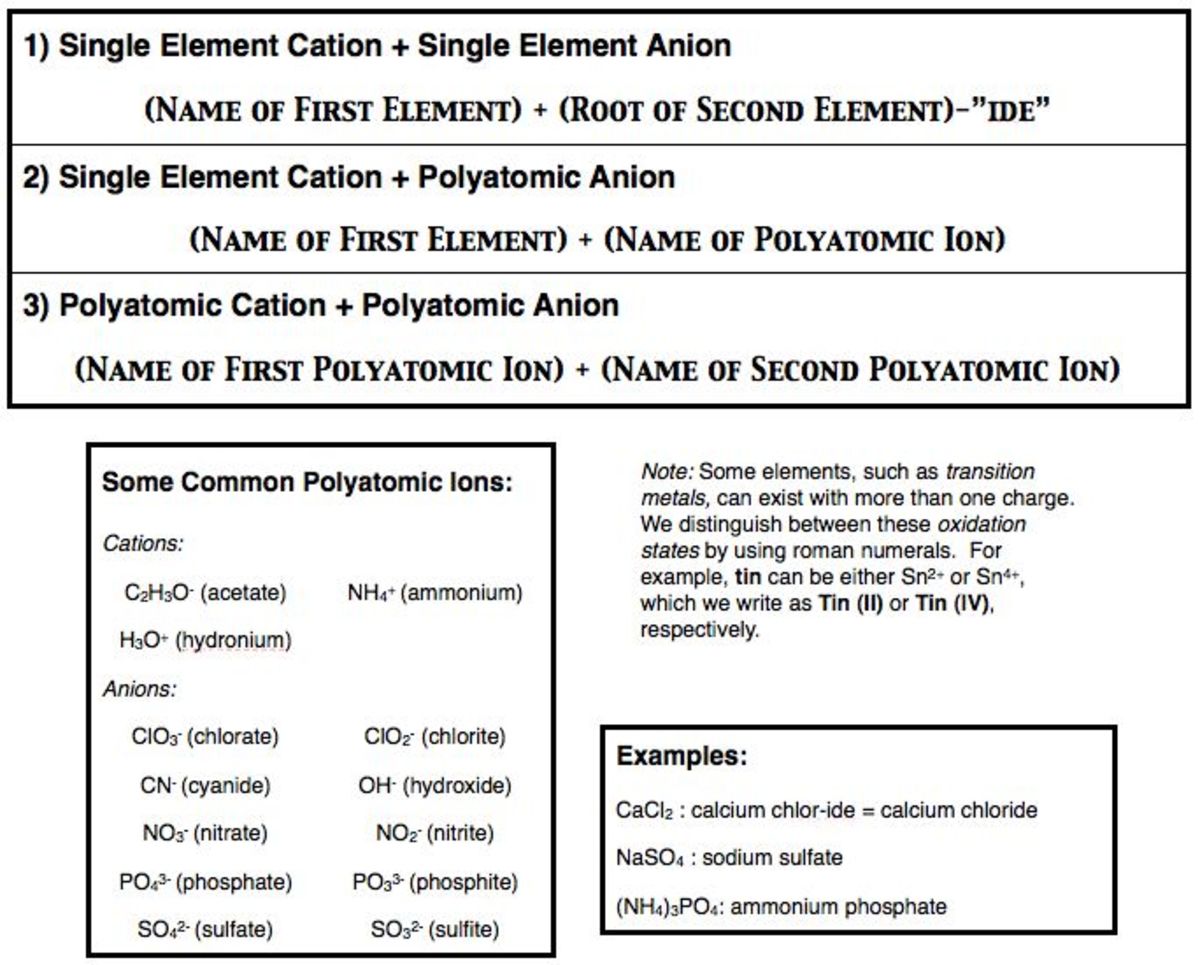
Ionic compounds involving polyatomic ions follow the same basic rule: Write the name of the metal first, and then simply add the name of the nonmetal (with the polyatomic anions, it is not necessary to add the -ide ending). How are ions named? An ionic compound is named first by its cation and then by its anion.
What is a polyatomic ion?
How many polyatomic ions are in chlorine?
What is the parenthesis for ammonium carbonate?
Is phosphoric acid an ous or ic?
How do polyatomic ions work in ionic compounds?
Ionic Compounds Containing Polyatomic Ions. Polyatomic ions are ions which consist of more than one atom. For example, nitrate ion, NO3-, contains one nitrogen atom and three oxygen atoms. The atoms in a polyatomic ion are usually covalently bonded to one another, and therefore stay together as a single, charged unit.
What are some uses of compounds with polyatomic ions?
Examples and UsesBaSO4 (barium sulfate) - Radiopaque medium in X-ray work, e.g. ulcers.CaSO4 (calcium sulfate) - Plaster casts.FeSO4 (iron(II) or ferrous sulfate) - For iron deficiency.MgSO4 (magnesium sulfate) - Cathartic.
How do you write formulas for compounds with polyatomic ions?
3:1911:20Writing Formulas with Polyatomic Ions - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo now I have the charges balanced. And now I want to write this in a chemical formula that showsMoreSo now I have the charges balanced. And now I want to write this in a chemical formula that shows how many of each I have to have in order to balance out the charges. So I need one calcium. So I'm
Is a polyatomic ion an ionic compound?
Polyatomic ions are ions. However, the atoms in polyatomic ions are held together by covalent bonds. Compounds containing polyatomic ions are ionic compounds.
How do you balance chemical equations with polyatomic ions?
0:113:59Balancing Chemical Equations with Polyatomic Ions - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo much easier tip number one keep polyatomic ions together if they are the same on both sides.MoreSo much easier tip number one keep polyatomic ions together if they are the same on both sides. Since so4 is the same on both the reactant. And product side then we keep them together o.
Why are polyatomic ions important?
Polyatomic ions are made up of more than one element and have an overall charge. Examples are SO42-, OH-, and NH4+. Polyatomic ions are important in chemistry because they are found in many common chemical compounds. Because of this, it is helpful to memorize the most common of these ions.
What are the rules for writing formulas for ionic compounds?
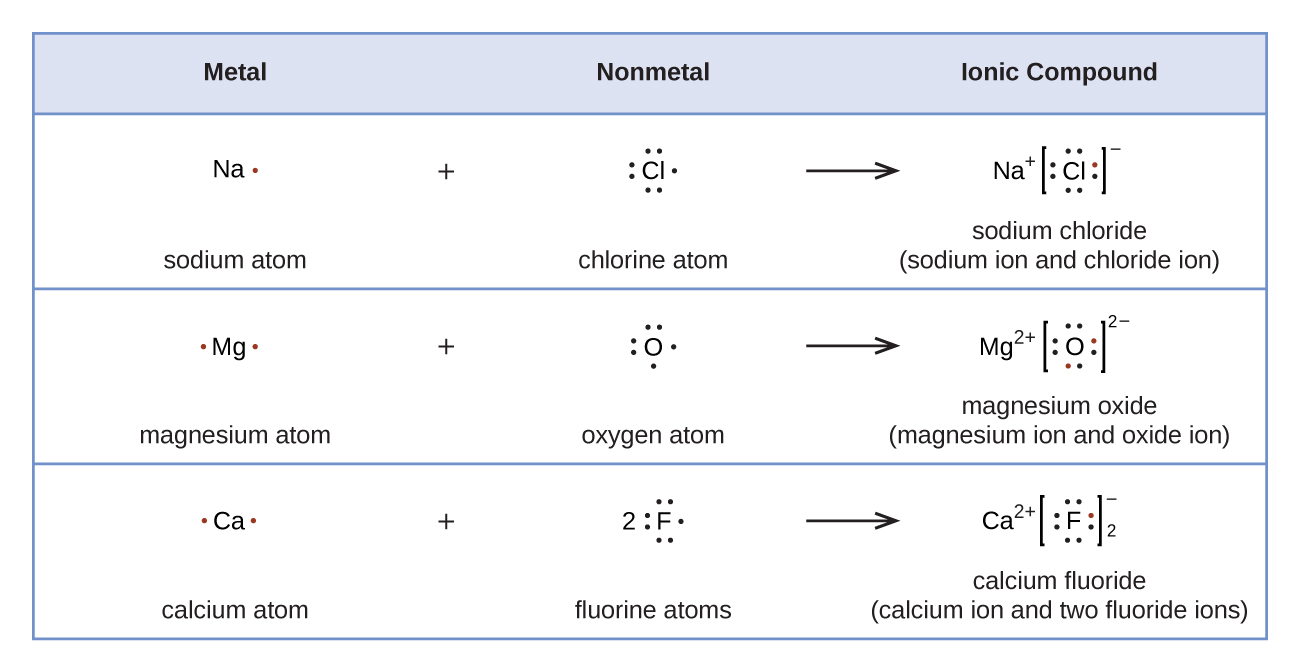
Writing Formulas of Ionic Compounds The cation is written first, followed by the monatomic or polyatomic anion. The subscripts in the formula must produce an electrically neutral formula unit. (That is, the total amount of positive charge must equal the total amount of negative charge.)
How do you write formulas for ionic compounds?
To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Then, identify the anion and write down its symbol and charge. Finally, combine the two ions to form an electrically neutral compound.
How do you name covalent compounds with polyatomic ions?
The naming conventions of polyatomic ions must either be remembered or be referred to when writing the formula of the compound. The first step is to identify the elemental cation and anion, and then name them. The cation is named first, then the second part of the name is the anion and its known or deduced charge.
How do you tell if a polyatomic ion is ionic or covalent?
0:003:30Do Polyatomic Ions have Ionic or Covalent Bonds? - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd here in the nitrate ion. These are covalent bonds as well. But what actually holds NH for two noMoreAnd here in the nitrate ion. These are covalent bonds as well. But what actually holds NH for two no. 3 in the big lattice structure the answer is that this on your shown bond. Here is ionic.
What bonds hold together polyatomic ions?
Recall that a polyatomic ion is a group of atoms that are covalently bonded together, and which carry an overall electrical charge. The ammonium ion, NH+4, is formed when a hydrogen ion (H+) attaches to the lone pair of an ammonia (NH3) molecule in a coordinate covalent bond.
How can a polyatomic ion be covalent and ionic at the same time?
Some chemical compounds contain both ionic and covalent bonds. These are ionic compounds that contain polyatomic ions. Often, a compound with both types of bonds contains a metal bonded to an anion of covalently bonded nonmetals.
What compounds contain polyatomic ions?
Indeed, most ionic compounds contain polyatomic ions. Well-known examples are sodium hydroxide (NaOH) with OH- as the polyatomic anion, calcium carbonate (CaCO3), and ammonium nitrate (NH4NO3), which contains two polyatomic ions: NH+ and NO3-.
What are examples of polyatomic ions?
Well-known examples of such polyatomic ions are the sulfate ion (SO42–), the hydroxide ion (OH–), the hydronium ion (H3O+), and the ammonium ion (NH4+).
What are polyatomic ions give two examples?
A polyatomic ion is a group of atoms carrying a charge (positive or negative). For example, nitrate ion (NO−3), hydroxide ion (OH - ).
What types of elements are found in polyatomic ions?
The polyatomic ion has two elements, Chlorine and Oxygen, in it. A good way to recognize that there are two elements in the polyatomic is to see that there are two capital letters in the ion's formula.
Why is it important to know the polyatomic ions?
Being familiar with the most common polyatomic ions will be helpful for recognizing ionic compounds and predicting their reactivity. While learning all the polyatomic ions can seem daunting, there are patterns to the formulas, names, and charges of many ions. These patterns can be learned, so you don't have to simply memorize all the ions.
What is a polyatomic ion?
In this article, we will discuss polyatomic ions. The prefix poly- means many, so a polyatomic ion is an ion that contains more than one atom. This differentiates polyatomic ions from monatomic ions, which contain only one atom. Examples of monatomic ions include , , , and many, many others. This article assumes you have a knowledge of basic monatomic ions as well as the conventions for naming ionic compounds and writing their chemical formulas.
How are ions formed?
Just as ions are formed when neutral atoms gain or lose electrons , a polyatomic ion is formed when a neutral molecule gains or loses electrons. Therefore, a polyatomic ion is a group of covalently bonded atoms that carries a net charge due to the fact that the total number of electrons in the molecule is not equal to the total number of protons in the molecule. In the Lewis dot structure of a polyatomic ion, the sum of the formal charges on all the atoms must equal the net charge on the ion.
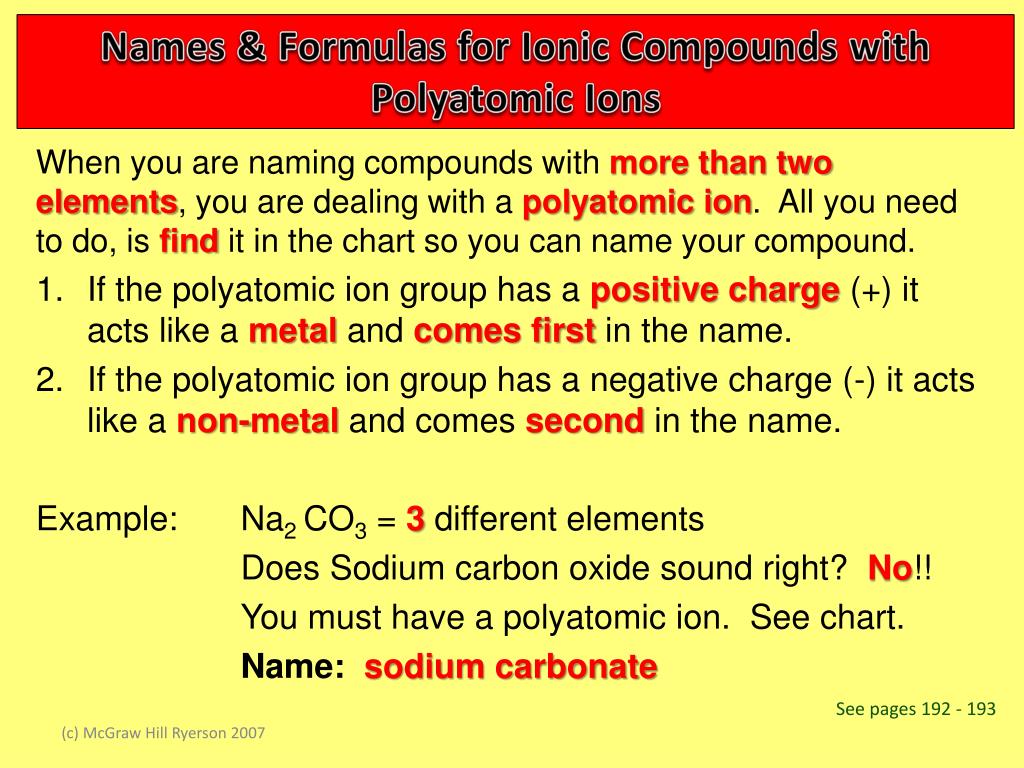
How to name an ionic compound?
When naming ionic compounds, it helps to first break down the formula into the cation (s) and the anion (s). In this compound, the cation is based on nickel. Nickel is a transition metal that can form multiple kinds of cations with different charges. That means we will need to figure out the charge on the nickel ion in this specific compound so that we can specify it when naming the ionic compound! Luckily, we know the charge on the anion: phosphate is a polyatomic ion that always has a charge of 3-. Since the overall charge for an ionic compound is zero, we can use the chemical formula and the charge on phosphate to calculate the charge on the nickel ion:
What is the game "Find the polyatomic ion"?
Bonus: Once you know the polyatomic ions, you can play the game, "Find the polyatomic ion," which involves looking for examples of compounds that contain polyatomic ions in everyday life. We gave examples of baking soda and chalk in this article. Can you find more? Hint: check out the ingredients list of your lotion, shampoo, or toothpaste. Feel free to post others in the comments below!
What is the overall charge of an ionic compound?
The overall charge for the ionic compound must be neutral, which means the sum of the charges from the cations and anions should add up to zero. We can use this rule to figure out the formula of an ionic compound when we know the charge on the anion and the cation. This rule can also be useful for deducing the charge of an ion when the chemical formula for the ionic compound is known.
How many lone pairs of electrons are in a polyatomic ion?
It contains one oxygen atom and one hydrogen atom. The single line between them represents the covalent bond, which contains two electrons shared between and . The dots around represent lone pairs of electrons. In hydroxide, the oxygen has three lone pairs of electrons, which makes for six lone pair electrons in total.
When writing formulas for compounds that have polyatomic ions, do you need to use a list of common?
When writing formulas for compounds that have Polyatomic ions, you need to use a list of common ions. This list is usually given to you by your instructor. Ask which of the Polyatomic ions you need to memorize and if you'll be able to use the list of the ions on an exam.
When writing formulas for ionic compounds with polyatomic ions, it is necessary to balance the charges between the?
Remember also, when writing formulas for ionic compounds with Polyatomic ions it is necessary to balance the charges between the metal and a Polyatomic ion.
What is polyatomic ion?
Polyatomic ions are ions made up of more than one atomic element. This example problem demonstrates how to predict the molecular formulas of several compounds involving polyatomic ions.
When writing a formula for an ionic compound, what is the first ion?
When you write the formula for an ionic compound, remember that the positive ion is always listed first. When there are two or more polyatomic ions in a formula, enclose the polyatomic ion in parentheses.
How to predict transition metals?
There is no simple way to predict the charges of the transition metals. Look on a table listing charges (valences) for possible values. For introductory and general chemistry courses, the +1, +2, and +3 charges are most often used.
What is a polyatomic ion?
First, let’s define a polyatomic ion. A polyatomic ion is a group of atoms covalently bonded together with a charge that affects the whole polyatomic unit. Some examples of polyatomic ions are in the following table. Make sure you memorize the names, formulas, and charges of each of the ions in this table.
How many polyatomic ions are in chlorine?
Note the halides, I, Cl, and Br, when combined with oxygen, can form four polyatomic ions. For Chlorine, the two additional ions are perchlorate ion, with four oxygens, and hypochlorite ion with only one oxygen. Please refer to the table for the names and formulas of the ions.
What is the parenthesis for ammonium carbonate?
For compounds that contain more than one polyatomic unit, use parenthesis. If we combine ammonium ion, (NH 4+) and carbonate ion (CO 32-) we have ammonium carbonate, (NH 4) 2 CO 3. Two ammonium ions are required for each carbonate ion.
Is phosphoric acid an ous or ic?
and phosphoric acid is formed. The acid must be neutral before naming it with the “ous” or “ic” suffix. More about naming acids in the next study guide.