What are the rules for electron configuration?
- Electrons must occupy the lowest available shell, closest to the nucleus.
- The maximum number of electrons that can fill each shell is: two in the first shell, eight in the second shell, eight in the third shell.
- Calcium, the 20 th element, has two further electrons that go in the fourth shell.
How to write shorthand electron configuration?
Writing Electron Configurations
- 1 s 2. The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron ...
- 1s2 2s1. ...
- Writing Electron Configurations – Examples. ...
- Writing Electron Configurations – Shorthand Method. ...
What is the Order of the electron configuration?
Electron configurations of atoms follow a standard notation in which all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence. For example, the electron configuration of sodium is 1s 2 2s 2 2p 6 3s 1. However, the standard notation often yields lengthy electron ...
How to find electron configuration?
Electron configurations help you to do this. To calculate an electron configuration, divide the periodic table into sections to represent the atomic orbitals, the regions where electrons are contained. Groups one and two are the s-block, three through 12 represent the d-block, 13 to 18 are the p-block and the two rows at the bottom are the f-block.
What is the i of a lanthanoid?
where i is a number between 0 and 14. Thus in the building-up process for the lanthanoids, electrons are being added to a subshell (4 f) whose principal quantum number is two less than that of the outermost orbital (6 s ). Addition of another electron to an inner shell buried as deeply as the 4 f has little or no effect on the chemical properties of these elements. All are quite similar to lanthanum (La) and might fit into exactly the same space in the periodic table as La. The lanthanoid elements are so similar to one another that special techniques are required to separate them. As a result, even approximately pure samples of most of them were not prepared until the 1870s. Following the element actinium (Ac) is a series of atoms in which the 5 f subshell is filling. The actinoids are somewhat less similar to Ac than the lanthanoids are to La because some exceptions to the usual order of filling orbitals occur in the case of Th, Pa, and U (Table 5.17. 1 ).
How does the 4S electron cloud affect the chemistry of transition metals?
The fact that the 4 s electron cloud is more extensive than the 3 d has an important influence on the chemistry of the transition elements. When an atom such as V (Figure 5.17. 1 ) interacts with another atom, it is the 4 s electrons extending farthest from the nucleus which first contact the other atom. Thus the 4 s electrons are often more significant than the 3 d in determining valence and the formulas of compounds. The 3 d electrons are “buried” under the surfaces of the atoms of the transition metals. Adding one more 3 d electron has considerably less effect on their chemical properties than adding one more 3 s or 3 p electron did in the case of the representative elements. Hence there is a slow but steady transition in properties from one transition element to another. Notice, for example, that except for Sc, all of the transition metals form chlorides, MCl2, where the metal has a valence of 2; examples are TiCl2, VCl2, CrCl2, and so on. This can be seen in the table found at the top of this page. The valence of 2 corresponds with the two 4s valence electrons.
What are the valences of transition metals?
Each of the transition metals also exhibits other valences where one or more of the 3 d electrons are also involved. For example, in some compounds V (vanadium) has a valence of 2 (VO, VCl 2) in others it has a valence of 3 (V 2 O 3, VCl 3 ), in still others it has a valence of 4 (VO 2, VCl 4 ), and in at least one case (V 2 O 5) it has a valence of 5. The chemistry of the transition metals is more complicated and a wider variety of formulas for transition-metal compounds is possible because of this variable valence. In some cases electrons in the d subshells act as valence electrons, while in other cases they do not. Although the 3 d electron clouds do not extend farther from the nucleus than 3 s and 3 p (and hence do not constitute another shell as the 4 s electrons do), they are thoroughly shielded from the nuclear charge and thus often act as valence electrons. This Jekyll and Hyde behavior of 3 d electrons makes life more complicated (and often far more interesting) for chemists who study the transition elements.
Why are lanthanoids and actinoids called inner transition elements?
Taken together, the lanthanoids and actinoids are called inner transition elements because the f subshells being filled lie so deep within the remaining electronic structure of their atoms.
What are the three periods of the periodic table?
The first three horizontal rows or periods in the modern periodic table consist entirely of representative elements. In the first period the distinguishing electrons for H and He are in the 1 s subshell. Across the second period Li and Be have distinguishing electrons in the 2 s subshell, and electrons are being added to the 2 p subshell in the atoms from B to Ne. In the third period the 3 s subshell is filling for Na and Mg, and therefore Al, Si, P, S, Cl, and Ar. As a general rule, in the case of the representative elements, the distinguishing electron will be in an ns or np subshell. The value of n, the principal quantum number for the distinguishing electron, can be quickly determined by counting down from the top of the periodic table. For example, iodine is a representative element in the fifth period. Therefore the distinguishing electron must occupy either the 5 s or 5 p subshell. Since I is on the right side of the table, 5 p is the correct choice.
How are valences predicted?
That is, the valences of the representative elements may be predicted on the basis of the number of valence electrons they have, or from the number of electrons that would have to be added in order to attain the same electron configuration as an atom of a noble gas . For representative elements the number of valence electrons is the same as ...
What is the relationship between electron configuration and the periodic table?
One more point needs to be emphasized about the relationship between electron configuration and the periodic table. The atoms of elements in the same vertical column of the table have similar electron configurations. For example, consider the alkaline-earth elements (group IIA). Using our rules for deriving electron configurations (Example 1) we have
How to determine electron configuration?
The electron configuration for a given element or atom can be determined using the periodic table. The periodic table consists of groups and periods. The periods, which are the horizontal rows that run left to right, tell us the principal energy level of an element. The groups, which are the vertical columns, tell us how filled each subshell is.
What does electron configuration tell you?
Electron configurations have a standard notation that tells you the principle energy levels and sublevels that electrons occupy. Here is the electron configuration for Helium:
What is an orbital diagram?
In an orbital diagram, orbitals are represented as boxes and electrons are represented by arrows (↑ or ↓), with two electrons occupying each orbital/box. Orbitals are labeled according to their principle energy levels and sublevels (1s, 2p, etc..). Helium, with two electrons in the 1s orbital has the following orbital diagram.
How many protons does carbon have?
Let’s start with finding the electron configuration for carbon (C). First, we need to know how many electrons carbon has. Since carbon has the atomic number six, it will also have six protons and six electrons. Furthermore, carbon is located in the second period and the second group in block p.
How many electrons does lithium have?
Lithium, containing three electrons, has two electrons occupying an s orbital at the first energy level, and one electron occupying an s orbital at the second energy level.
Why is Lithium's configuration written using only 1s 2?
A Note: Lithium’s configuration is written using only “1s 2 ” and not “1s 1 1s 2 ” because between Hydrogen and Helium, the energy level and orbital do not change. Only the electron occupancy changes, which we denote by changing the superscript from 1 to 2.
What are the four subshells of an atom?
Furthermore, there are subshells. The four subshells you will work with the most in chemistry are the s,p,d, and f subshells.
What are the electronic configurations of elements in the periodic table?
What are the electronic configurations of elements in the periodic table?
Why is it important to know how electrons are distributed in an atom for chemistry purposes?
It’s important to know where an electron might be found within an atom so as not to confuse it with another element when analyzing a compound or writing chemical formulas
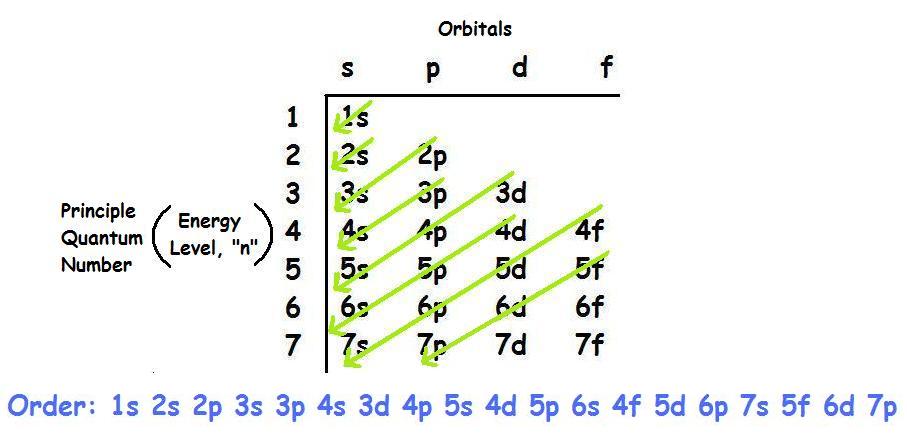
What is the Aufbau principle?
The Aufbau principle, is the idea that electrons occupy orbitals of lower energy before occupying higher-energy orbitals. Electrons are filled in order from 1s to 7p and an illustration can be seen below:
How are electronic configurations determined?
Electronic Configurations are determined by the number of electrons within an atom’s orbitals and their relative energy levels. The shells are occupied one level at a time; first shell (K), second shell (L), third shell (M) etcetera until you reach nine where there is no further Shells. As each new shell is filled, electrons will drop to the next lower energy level.
Why are electrons more stable?
The electrons in an atom are considered more stable when they’re “bonding orbitals” because this is where there’s no net charge and the electron distribution is symmetrical; if you had to choose between bonding or antibonding, it would always be better for the electrons to be in a bonding orbital.
How many unpaired electrons are in an atom?
Electrons in an atom are distributed according to the electrons’ stability. Usually, atoms will have one or two unpaired electrons. The distribution of these unpaired electrons is called electron configuration and each type of arrangement has a name:
Why are electron configurations important?
Electron configurations are a very important factor in determining the chemical properties of an atom. The outermost electrons around an atom determine where it can and cannot form bonds with other atoms, which determines its stability as well as what kind of reactions it will undergo. For example:
How are electron configurations for cations made?
First you should write their normal electron configuration and then when you remove electrons you have to take them from the outermost shell. Note that this is not always the same way they were added.
What is electron configuration?
Electron configurations are the summary of where the electrons are around a nucleus. As we learned earlier, each neutral atom has a number of electrons equal to its number of protons. What we will do now is place those electrons into an arrangement around the nucleus that indicates their energy and the shape of the orbital in which they are located. Here is a summary of the types of orbitals and how many electrons each can contain:
How many electrons are needed to fill an orbital?
Here is a summary of the types of orbitals and how many electrons each can contain: So based on what we know about the quantum numbers and using the chart above, you need 2 electrons to fill an s orbital, 6 electrons to fill a p orbital, 10 electrons to fill a d orbital and 14 electrons to fill the f orbital.
Why is the periodic table aligned?
The reason this was done is that the configuration of an element gives the element its properties and similar configurations yield similar properties.
Why is electronegativity important?
Electronegativity is an atoms ability to pull electrons towards itself.
What is the order in which electrons are placed into the orbitals?
Order of Fill. The order in which electrons are placed into the orbitals is based on the order of their energy. This is referred to as the Aufbau principle. The lowest energy orbitals fill first. Just like the quantum numbers themselves this order was determined by calculation and is summarized by the following chart:
How many electrons does oxygen have?
With 10 electrons you should note that oxygen's electron configuration is now exactly the same as Neon's. We talked about the fact that ions form because they can become more stable with the gain or loss of electrons to become like the noble gases and now you can actually see how they become the same.
How many electrons can an orbital hold?
Each orbital can hold a maximum of 2 electrons, which means s orbitals can go up to 2, p orbitals up to 6 (because 2 electrons max for 3 orbitals), d orbitals can go up to 10, and f orbitals can go up to 14.
How many orbitals are there in period 4?
Because there are 5 possible ml values, there are 5 d orbitals per energy level (starting from period 4), but the energy level for d orbitals is 1 less than the period number, which is why period 4 elements have 3d orbitals, period 5 have 4d orbitals, etc.
How many superscripts are there for a s orbital?
Notice in these examples that the superscripts for s orbitals only go up to 2, for p got up to 6, for d go up to 10, and for f go up to 14. This is due to the number of possible ml values for that orbital type.
How many quantum numbers are there in an element?
Every single electron in an element has a different set of 4 quantum numbers; no 2 electrons have the same combination of quantum numbers!
What is the first quantum number?
The first quantum number is the principal quantum number n , which tells you the energy of those electrons. n ranges from 1 to 7 (theoretically can go 8 +) .
Why are periodic trends important?
Periodic trends are very useful when identifying and classifying elements.
What is the third number in the magnetic field?
The third number, the magnetic quantum number ml, tells you how many orbitals are in each subshell. They range from −l to l. For example, an electron in an s orbital on would have an l of 0 and therefore can have only ml value of 0. There is only 1 possible ml so there can only be 1 s orbital per energy level.
How to write out the electron configuration of an element?
Generally speaking, if you want to write out the ground-state electron configuration of an element, you work your way up to the element’s atomic number, adding and filling sublevels as you encounter them.
Why do chemists use electron configurations?
Chemists use electron configurations to describe the arrangement of electrons around the nucleus of atoms. This helps in predicting how atoms will join together to from chemical bonds, and their behavior.
How are electrons determined?
The electron configuration of each element is unique to its position on the periodic table. The energy level is determined by the period and the number of electrons is given by the atomic number of the element. Orbitals on different energy levels are similar to each other, but they occupy different areas in space.
How many electrons can an orbital hold?
The four different types of orbitals (s,p,d, and f) have different shapes, and one orbital can hold a maximum of two electrons. The p, d, and f orbitals, however, have different sub-levels, thus can hold more electrons. The electron configuration of an atom is the representation of the arrangement of electrons d.
What are the determining factors of the unique chemistry of an element?
Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. Electrons in the outermost shell - valence electrons - are the determining factor for the unique chemistry of the element.
Why do I put 8 after 18 electrons in the consecutive shells?
A question may arise in your mind that why I placed 8 after 18 electrons in the consecutive shells this is because to obtain the Valency of the element, this can be done in normal conditions but the inner shell must contain at least 8 electrons or till the capacity it can hold the number of electrons.
Where are electrons found in an atom?
Electrons exhibit a negative charge and are found around the nucleus of the atom in electron orbitals, defined as the volume of space in which the electron can be found within 95% probability. The four different types of orbitals (s,p,d, and f) have different shapes, and one orbital can hold a maximum of two electrons.
What is the electron configuration of H and He?
Let us start with H and He. Their electron configurations are 1 s 1 and 1 s 2 , respectively; with He, the n = 1 shell is filled. These two elements make up the first row of the periodic table (Figure 9.7. 2)
What is the role of electrons in the periodic table?
The arrangement of electrons in atoms is responsible for the shape of the periodic table. Electron configurations can be predicted by the position of an atom on the periodic table.
Why does the periodic table have the structure it does?
Why does the periodic table have the structure it does? The answer is rather simple, if you understand electron configurations: the shape of the periodic table mimics the filling of the subshells with electrons.
How many electrons are in the 3D subshell?
After the 4 s subshell is filled, the 3 d subshell is filled with up to 10 electrons. This explains the section of 10 elements in the middle of the periodic table (Figure 9.7. 8 ).
What is the periodic table?
Previously, we introduced the periodic table as a tool for organizing the known chemical elements. A periodic table is shown in Figure 9.7. 1. The elements are listed by atomic number (the number of protons in the nucleus), and elements with similar chemical properties are grouped together in columns.
Which subshell is being occupied with electrons?
For the next six elements, the 2 p subshell is being occupied with electrons. On the right side of the periodic table, these six elements (B through Ne) are grouped together (Figure 9.7. 4 ).
Do all elements have the same chemistry?
They all have a similar electron configuration in their valence shells: a single s electron. Because much of the chemistry of an element is influenced by valence electrons, we would expect that these elements would have similar chemistry— and they do . The organization of electrons in atoms explains not only the shape of the periodic table, but also the fact that elements in the same column of the periodic table have similar chemistry.