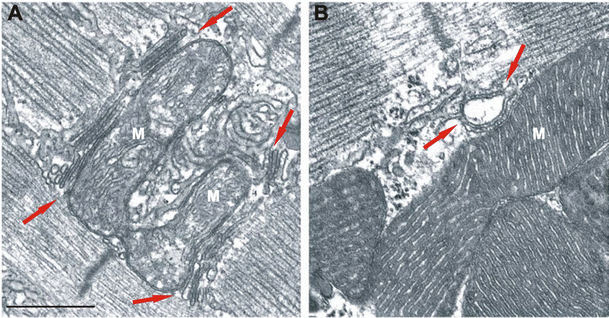
How is acetyl-CoA made in the mitochondria?
Acetyl-CoA is made in the mitochondria by metabolizing fatty acids, and the oxidation of pyruvate of acetyl-CoA. When the body has an excess of ATP, the energy in acetyl-Coa can be stored in the form of fatty acids. Acetyl-CoA must cross the mitochondrial membrane to the cytosol, where fatty acid synthesis takes place.
How is acetyl CoA obtained?
-Acetyl CoA can be obtained from the metabolism of carbohydrates, fatty acids, and amino acids. glucose undergoes glycolysis, its product, pyruvate, enters the mitochondrion via active transport and is oxidized and decarboxylated. What is Acetyl-CoA composed of?
What is the role of acetyl CoA in mitochondria?
As a result, acetyl-CoA is generated in the mitochondria for oxidation or other possible fates. In the liver, mitochondrial acetyl-CoA is used to synthesize ketone bodies (acetoacetate and β-hydroxybutyrate) as alternative fuel sources for the brain and heart under conditions of carbohydrate scarcity [13,16].
Why do acetyl-CoA levels decrease in the mitochondria?
Instead of shipping acetyl units out to the cytosol, there is now a greater requirement for acetyl-CoA to be oxidized in the mitochondria for ATP synthesis (Fig. 1). Under such conditions, nucleocytosolic acetyl-CoA levels therefore decrease. Fatty acids are a significant source of this mitochondrial acetyl-CoA pool [13].

What is the role of acetyl-coa in protein deacetylases?
The accumulation of acetyl-CoA in subcellular compartments may also necessitate the activity of deacetylase enzymes to remove non-enzymatic acetylation modifications that could intentionally or unintentionally compromise protein function [28,53,54]. Such a “repair” or “detoxification” role may be fulfilled by the sirtuin family of protein deacylases (Fig. 2). Consistent with this idea, hyperacetylation of mitochondrial enzymes occurs in the absence of mitochondrial SIRT3 [55–57], and deacetylation of these enzymes typically increases their activity [53]. Moreover, the expression of SIRT3 is increased specifically under fasting states, in response to high-fat diets, or during exercise - conditions that all promote increased mitochondrial acetyl-CoA [53]. Likewise, the potential of proteins to be modified by other acyl-CoA metabolites besides acetyl-CoA is supported by the discovery of a wide variety of acylation modifications present on proteins, along with associated sirtuins that preferentially catalyze their removal [58–61]. Evidence that sirtuins evolved specifically to remove non-enzymatic protein acylation as a form of protein quality control has been summarized in a recent review [54]. In this model, failure of sirtuins to remove aberrant acylation modifications would hinder the function of effected proteins and consequently lead to dysfunctions in metabolism and susceptibility to disease [47,55,57].
How might cells actually sense the abundance of acetyl-CoA?
How might cells actually sense the abundance of acetyl-CoA? It is perhaps no coincidence that acetyl-CoA doubles as the acetyl donor for protein acetylation modifications (including histone acetylation) (Fig. 2). The abundance of protein acetylation modifications could therefore reflect the cell’s metabolic state to regulate various protein activities. Studies performed under carbon-rich conditions where acetyl-CoA synthesis is not limiting may mask the contributions of this metabolite in cellular regulation. However, most organisms, as well as particular tissue microenvironments in vivoexperience challenges in the nutrient environment that might limit acetyl-CoA biosynthesis or availability (e.g., carbon starvation or hypoxia). Recent studies have begun to provide compelling evidence that many protein acetylation modifications are indeed modulated by acetyl-CoA availability [27,28].
What happens to acetyl-CoA during starvation?
During starvation, cells must typically shift from growth to survival mode and alter metabolism towards functions important for viability. Instead of shipping acetyl units out to the cytosol, there is now a greater requirement for acetyl-CoA to be oxidized in the mitochondria for ATP synthesis (Fig. 1). Under such conditions, nucleocytosolic acetyl-CoA levels therefore decrease. Fatty acids are a significant source of this mitochondrial acetyl-CoA pool [13]. CoA synthesis is induced to activate fatty acids as fatty acyl-CoAs [14,15], which can then be transported into mitochondria via the carnitine shuttle for β-oxidation. As a result, acetyl-CoA is generated in the mitochondria for oxidation or other possible fates. In the liver, mitochondrial acetyl-CoA is used to synthesize ketone bodies (acetoacetate and β-hydroxybutyrate) as alternative fuel sources for the brain and heart under conditions of carbohydrate scarcity [13,16]. Under such conditions, lower nucleocytosolic acetyl-CoA will also limit fatty acid synthesis, histone acetylation, and other growth-related processes. ATP citrate lyase is inhibited under these situations at both the transcriptional and post-translational levels [17,18].
What is acetyl-CoA used for?
Nucleocytosolic pools of acetyl-CoA are also utilized for histone acetylation and the activation of gene expression. ATP citrate lyase was shown to provide a source of acetyl-CoA for histone acetylation in mammalian cells [9]. The budding yeast Saccharomyces cerevisiae, which lacks ATP citrate lyase, relies on acetyl-CoA synthetase enzymes to supply acetyl-CoA for histone acetylation [10]. Moreover, a special cohort of yeast genes important for growth, such as those required for ribosome biogenesis and the G1 cyclin CLN3, are especially dependent on histone acetylation for their activation [11,12]. As such, the expression of these growth genes is closely coupled to acetyl-CoA as an indicator of the cell’s nutritional state. Thus, when carbon sources are abundant, nucleocytosolic amounts of acetyl-CoA accumulate and facilitate the processes of lipid synthesis and histone acetylation (Fig. 1).
What is YMC in yeast?
The yeast metabolic cycle (YMC) offers a system to investigate whether particular acetylation modifications might be coupled to acetyl-CoA itself. Studies of yeast cells undergoing the YMC during continuous, glucose-limited growth in a chemostat have revealed periodic changes in intracellular acetyl-CoA amounts as yeast cells alternate between growth and quiescent-like phases [22]. Several proteins are dynamically acetylated precisely in phase with the observed acetyl-CoA oscillations [11]. These include histones, several components of the transcriptional coactivator SAGA, a subunit of the SWI/SNF chromatin remodeling complex Snf2p, and a transcriptional coactivator of ribosomal subunit gene expression Ifh1p [11,41]. Interestingly, the dynamic acetylation of all of these proteins is dependent on the acetyltransferase Gcn5p, suggesting this enzyme has the capability of acetylating its substrates in tune with acetyl-CoA fluctuations in vivo. Consistent with this hypothesis, mutations within Gcn5p slow growth, disrupt the yeast metabolic cycle, or alter the cell’s responsiveness to acetate [11,12]. Moreover, acetylation of SAGA subunits appears to aid its recruitment to growth genes [11]. A brief survey of other acetylated proteins that are not known to be Gcn5p substrates showed they are not dynamically acetylated across the YMC [11]. An analysis of the genomic regions bound by these acetylated histones revealed that several marks, in particular H3K9Ac, were present predominantly at growth genes, specifically during the growth phase of the YMC when acetyl-CoA levels rise [11,25]. These considerations suggest that the acetylation of these nuclear-localized proteins collectively functions to promote the activation of growth genes in response to a burst of nucleocytosolic acetyl-CoA.
What is publisher's Disclaimer?
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Why do metabolites feed back?
It has become evident that metabolites themselves must feed back to regulate gene expression, signal transduction, and various protein activities in cellular decision-making processes [1,2]. These small molecule metabolites play critical roles in relaying metabolic information to their protein and nucleic acid counterparts. However, despite increased recognition of such reciprocal interplay, many aspects of the mechanisms through which metabolites exert their influence on cellular regulatory mechanisms are still being unraveled.
How many molecules of ATP does a citric acid molecule produce?
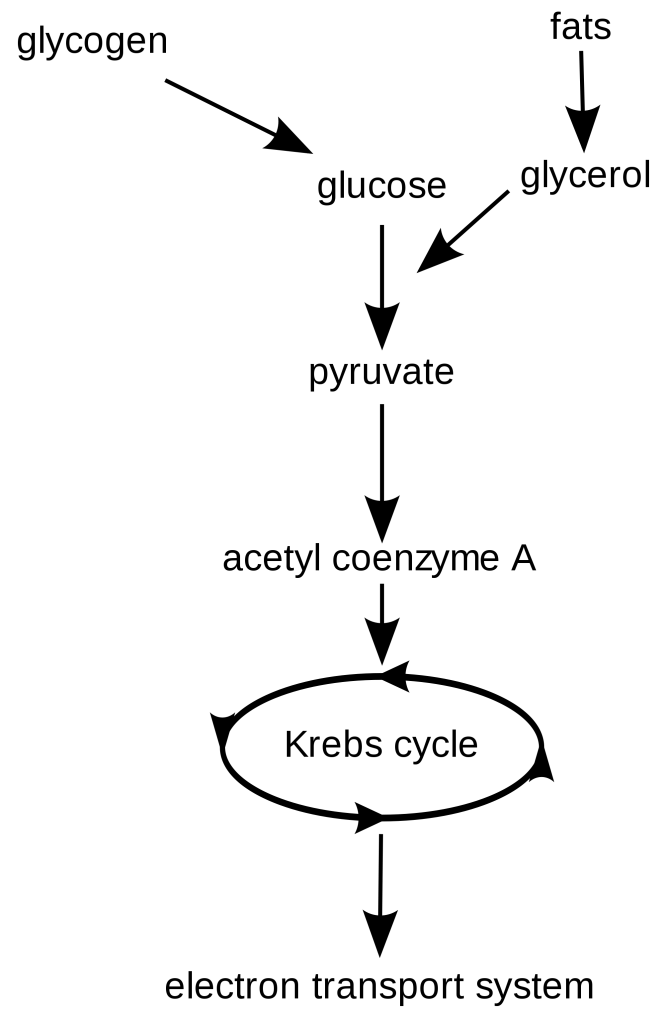
The citric acid cycle constantly forms and regenerates coenzyme A and acetyl-CoA. A single molecule of acetyl-CoA will produce 10 to 12 molecules of ATP. Where the acetyl group has been released from acetyl-CoA, the remaining coenzyme A aids in the conversion of pyruvate to acetyl CoA before re-entering the citric acid cycle.
What happens when pantothenate levels are low?
When pantothenate levels in the body are low, CoA and acetyl-CoA levels will also be low. As CoA production overlaps with other vitamin-producing pathways, these can also affect the availability of both CoA and acetyl-CoA. Examples of competing vitamins are folic acid and thiamine. Pantothenate.
What is acetyl coenzyme A?
Acetyl-CoA or acetyl coenzyme A is a component of cellular respiration (energy conversion) that adds acetyl groups to biochemical reactions. These reactions are used in the metabolizing of proteins, carbohydrates, and lipids that will provide energy sources in the forms of adenosine triphosphate (ATP), lactic acid, and ketone bodies.
What is the role of acetyl co-A?
Its primary job is to transfer the carbon atoms in acetyl to other molecules. The components of acetyl co-A are, not surprisingly, acetyl and coenzyme A. An acetyl group is represented by the chemical formula CH 3 CO. Acetyl is produced by the breakdown of pyruvate, a derivative of carbohydrate.
How is acetyl produced?
Acetyl is produced by the breakdown of pyruvate, a derivative of carbohydrate. When pyruvate breaks down, it produces small bonded carbon molecules (C 2 ). When they react with CoA, the combined molecule becomes acetyl-CoA. Coenzyme A is a cofactor – it assists an enzyme to provide an effect.
How many hydrogen ions are produced in a glycolysis reaction?
In simplified terms, a glycolysis reaction produces two hydrogen ions, a total gain of two ATP molecules, and two each of water and pyruvate molecules from a single glucose molecule (C₆H₁₂O₆). C 6 glucose becomes two C 3 pyruvate molecules.
What is the second step of glucose metabolism?
The second step of glucose metabolism depends upon the presence or absence of oxygen or the ability of the cells to use it. Where no or limited oxygen is available, pyruvate travels an anaerobic pathway that leads to lactic acid production ( anaerobic respiration ).