
By binding substrates to their active sites, enzymes stabilize the structure of the transition state. This in turn lowers of the free energy of the transition state, which in turn decreases the rate of the chemical reaction. Enzymes do not however change the Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy (IUPAC recommended name: Gibbs energy or Gibbs function; also known as free enthalpy to distinguish it from Helmholtz free energy) is a thermodynamic potential that measures the maximum or reversible work that may be performed by a thermodynamic system at a constant temperature and pressure (isothermal, isobaric).
What is the transition state of an enzyme?
Application to EnzymesEdit. The enzyme's ability to make the reaction faster depends on the fact that it stabilizes the transition state. The transition state's energy or, in terms of a reaction, the activation energy is the minimum energy that is needed to break certain bonds of the reactants so as to turn them into products.
How do enzymes lower the energy of a reaction?
Enzymes work in several ways to lower the energy needed to complete a reaction. One method is to lower the energy of the transition state, during which the reactants are breaking and forming chemical bonds.
How do enzymes change the equilibrium of a reaction?
Enzymes don’t change the equilibrium between the reactants and products of a reaction, but do lower the transition state energy so that the reaction becomes more likely and happens faster.
How does a catalyst affect the activation energy of an enzyme?
So with the catalyst, the activation energy barrier that molecule A has to overcome in order to get to point B is much smaller. And this will mean that your reaction will have a transition state with a much lower energy, meaning that it's more stable with the enzyme and also that the reaction as a whole have a much lower activation energy.
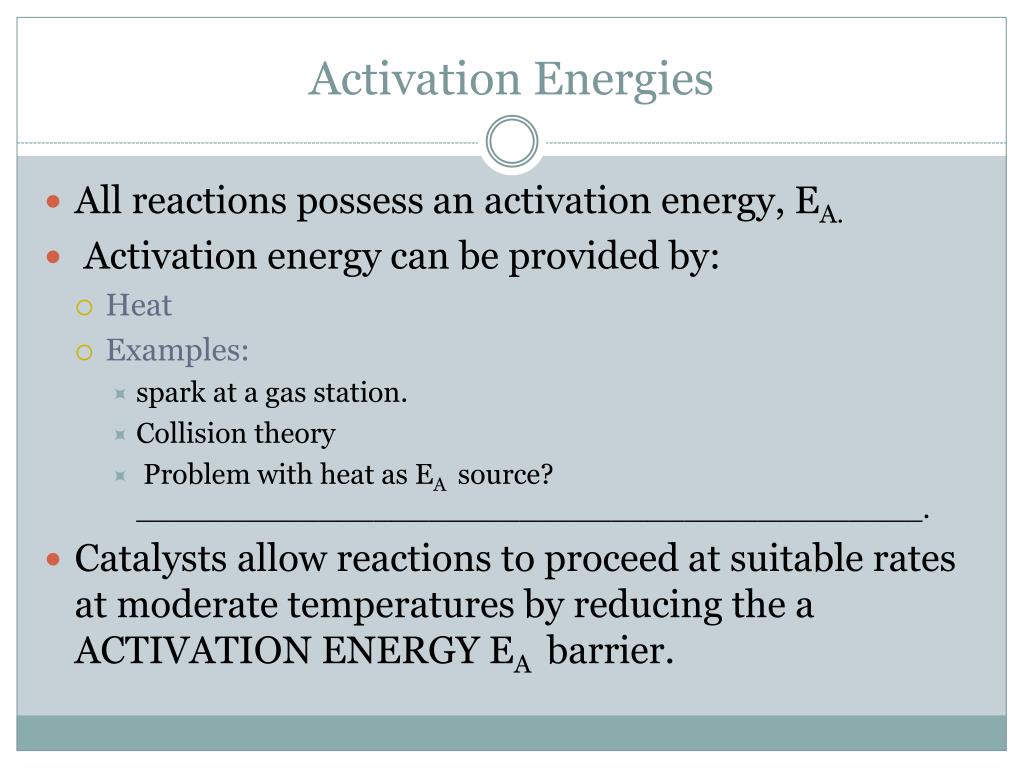
How do enzymes affect activation energy and the transition state?
Lowering the Activation Energy The catalyst speeds up the rate of reaction without being consumed; it does not change the initial reactants or the end products. Enzymes (blue line) change the formation of the transition state by lowering the energy and stabilizing the highly energetic unstable transition state.
How do enzymes lower the energy of the transition state?
One method is to lower the energy of the transition state, during which the reactants are breaking and forming chemical bonds. Enzymes do this by offsetting the distribution of electrical charges surrounding the transition state reactant/product complexes.
How does an enzyme affect energy?
Enzymes are biological catalysts. Catalysts lower the activation energy for reactions. The lower the activation energy for a reaction, the faster the rate. Thus enzymes speed up reactions by lowering activation energy.
How do enzymes induce the transition state?
Intermolecular interactions between the enzyme and substrate induce a new fit that facilitates the formation of a transition state and results in the catalysis of the reaction. The reaction follows the standard flow where the Enzyme (E) and the Substrate (S) interact to form an Enzyme-Substrate Complex (ES).
Why do enzymes bind to transition state better?
7:5213:30Enzymes Stabilize Transition State - YouTubeYouTubeStart of suggested clipEnd of suggested clipThese partially broken foreign bonds it lowers. The energy of activation. It stabilizes theMoreThese partially broken foreign bonds it lowers. The energy of activation. It stabilizes the transition state lowering that free energy of activation. And if we lower that free energy of activation.
Do enzymes increase or decrease activation energy?
Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. The increased rate is the same in both the forward and reverse directions, since both must pass through the same transition state.
What is the transition state in an enzyme catalyzed reaction?
The transition state is the state corresponding to the highest energy along the reaction coordinate. It has more free energy in comparison to the substrate or product; thus, it is the least stable state. The specific form of the transition state depends on the mechanisms of the particular reaction.
What happens when the transition state is reached?
The transition state is a high-energy state, and some amount of energy – the activation energy – must be added in order for the molecule reach it. Because the transition state is unstable, reactant molecules don't stay there long, but quickly proceed to the next step of the chemical reaction.
What is binding energy and how does it contribute to enzyme specificity?
The binding energy is the free energy that is released by the formation of weak interactions between a complementary substrate and enzyme. The binding energy is maximized since only the correct substrate can interact with an enzyme and is released when the enzyme facilitates formation of the transition state.
What is the role of transition state stabilization in enzyme catalysis?
Transition-state stabilization compared to free energy during an enzymatic reaction. (a) The transition-state stabilization proposal suggests a thermodynamic equilibrium in which tight binding of reactants (S) to the enzyme (E) at the transition state (‡) is proportional to enzymatic rate enhancement (kenz/kchem).
Does enzyme bind substrate or transition state more tightly?
a) The active site of an enzyme binds the substrate of the reaction it catalyses more tightly than it does the transition state intermediate.
Do enzymes lower the free energy of a reaction?
Well, first we learned that enzymes work by lowering the free energy of activation of a reaction, making it much easier for the reactants to transition and form products.
Do enzymes lower the Gibbs free energy of a reaction?
Enzymes do not affect the Gibbs free energy of a reaction. That means that they do not increase or decrease how much products are formed and how much reactants are used up nor do they increase or decrease the free energy values of the products and reactants.
Do enzymes decrease entropy?
Two ways that an enzyme could diminish the TΔS⧧ penalty. (a) If the entropy loss in water is dominated by ordering the reactants, the enzyme pays this penalty upon substrate binding, and the reaction can proceed without further entropy loss.
When are new enzymes needed?
Thus, new enzymes are needed when these enzymes 'age' . Also take note that enzymes are well regulated thus when there is a surmounting amount of it, the body reacts via negative feedback and inhibits its formation.
Do enzymes control reactions?
Enzymes also control reactions.
Does an enzyme make a spontaneous reaction happen faster?
The presence of an enzyme will, however, make a spontaneous reaction occur faster. The occurrence of a reaction randomly or it's requirement for an enzyme is a factor of how much energy is needed to overcome the activation energy barrier. Comment on Iman Baharmand's post “Hi there!
Do enzymes have short half lives?
Direct link to Alessandro.M.Rosa's post “Enzymes generally have very short half-lives, some...”. more. Enzymes generally have very short half-lives, some on the order of minutes. While they are not degraded in the reactions themselves, their structural stability is such that they break down rapidly.
Does an enzyme lower activation energy?
The enzyme does not lower the activation energy, what it does is provide an alternate route that is at a lower energy level, thus more molecules are able to react. Now those at a higher energy can still react via the route without the enzyme, but can also go the route of lower energy through use of the enzyme. 5 comments.
Does spontaneity affect the enzymes?
Great question; it's important to realize that spontaneity is a factor of a reactions 'Thermodynamics'. Whereas as an enzyme effects a reaction's 'Kinetics'. That is to say, an enzyme will lower a reaction's activation energy (EA) but it will not necessarily make a reaction happen spontaneously.
How do enzymes help a reaction?
Enzymes are usually proteins that act like catalysts. The enzyme's ability to make the reaction faster depends on the fact that it stabilizes the transition state. The transition state's energy or, in terms of a reaction, the activation energy is the minimum energy that is needed to break certain bonds of the reactants so as to turn them into products. Enzymes decreases activation energy by shaping its active site such that it fits the transition state even better than the substrate. When the substrate binds, the enzyme may stretch or distort a key bond and weaken it so that less activation energy is needed to break the bond at the start of the reaction. In many cases, the transition state of a reaction has a different geometry at the key atom (for instance, tetrahedral instead of trigonal planar). By optimizing binding of a tetrahedral atom, the substrate is helped on its way to the transition state and therefore lowers the activation energy, allowing more molecules to be able to turn into products in a given period of time. The enzyme stabilizes the transition state through various ways. Some ways an enzyme stabilizes is to have an environment that is the opposite charge of the transition state, providing a different pathway, and making it easier for the reactants to be in the right orientation for reaction.
What is the transition state of a chemical reaction?
By definition, the transition state is the transitory of molecular structure in which the molecule is no longer a substrate but not yet a product. All chemical reactions must go through the transition state to form a product from a substrate molecule. The transition state is the state corresponding to the highest energy along the reaction coordinate. It has more free energy in comparison to the substrate or product; thus, it is the least stable state. The specific form of the transition state depends on the mechanisms of the particular reaction.
What is the transition state analog?
In 1948, Linus Pauling proposed that transition state analogs should be effective inhibitors of enzymes. These molecules are mimics of transition states of the substrate of a particular enzyme reaction. Because they are so similar to the transition states of the substrate, they can bind to the enzyme, oftentimes much more tightly than the substrate can. The fact that these transition state analogs bind so tightly to enzymes makes it an effective enzyme inhibitor. The transition state theory says that the occurrence of enzymatic catalysis is equivalent to an enzyme binding to the transition state more strongly than it binds to the ground-state reactants. This theory is based on the two fundamental principles of physical chemistry: Absolute reaction-rate theory and the thermodynamic cycle. Also, the thermodynamic cycle relating substrate binding and transition state binding apply elementary transition-state theory to enzymatic catalysis, which is a restatement of Pauling's description of transition-state binding in quantitative symbols. He has stated that the catalytic powers of enzymes result from their highly specific binding of the transition state.
What is the role of water in peptide hydrolysis?
In a normal peptide hydrolysis reaction without the help of a catalyst, water acts as a nucleophile to attack the electrophilic carbonyl carbon. The carbon atom being attacked goes from its initial sp2 state (trigonal planar) to a new sp3 state (tetrahedral) in its transition state.
How do enzymes affect the energy of a chemical reaction?
When they act on chemical reactions, enzymes do not change the Gibbs free energy, which means they do not increase or decrease how much products are formed at the end of that reaction.
How do enzymes lower activation energy?
This lecture will look at how enzymes work and the process of enzymes lower activation energy. The enzyme reacts with substrate and makes the products, and the substrate is kind of complementary towards the enzyme active site.
What is the key aspect of the enzyme-substrate reaction?
So the key aspect of the enzyme-substrate reaction is that the enzyme reduces the activation energy for this reaction, thereby augmenting the rate. The binding energy provides this. The substrate is bound with an enzyme. In the active site, there are several interactions.
Why is activation energy needed?
Activation energy is needed to break down chemical bonds allowing the reaction to occur. When an enzyme binds to a substrate, it lowers the substrate molecules’ energy to react to form products. This increases the chance of the reaction occurring and therefore increases the rate of reaction.
What is the difference between the energy of the reactant and the transition state?
The difference between the energy of the reactant and the transition state is the activation energy. So activation energy without the enzyme kind of looks like the black curve. If you have an enzyme that can catalyze this reaction, the graph looks like the red curve.
Why is activation energy reduced in enzyme-substrate reaction?
This reduction in the activation energy is the key aspect of the enzyme-substrate reaction because it reduces the active activation energy.
What is the role of enzymes in a reaction?
If the reaction were to happen spontaneously, an enzyme’s role is to lower the activation energy needed to start a reaction to proceed quickly without a temperature change. Make sure that our body does not change in temperature.
What happens to enzymes as temperature increases?
As temperature increases, more enzymes' molecules' Active Sites' shapes will be less Complementary to the shape of their Substrate, and more enzymes will be Denatured. This will decrease the rate of reaction.
How does an enzyme affect its activity?
The activity of an Enzyme is affected by its environmental conditions. Changing these alterthe rate of reaction caused by the enzyme. In nature, organisms adjust the conditions of their enzymes to produce an Optimum rate of reaction, where necessary, or they may have enzymes which are adapted to function well in extreme conditions where they live.
What is the temperature at which the maximum rate of reaction occurs?
The temperature at which the maximum rate of reaction occurs is called the enzyme's Optimum Temperature. This is different for different enzymes. Most enzymes in the human body have an Optimum Temperature of around 37.0 °C.
How does the activity of an enzyme affect its environment?
The activity of an Enzyme is affected by its environmental conditions. Changing these alter the rate of reaction caused by the enzyme. In nature, organisms adjust the conditions of their enzymes to produce an Optimum rate of reaction, where necessary, or they may have enzymes which are adapted to function well in extreme conditions where they live.
How does an enzyme work?
An enzyme works by lowering the Activation energy of the chemical reaction it catalyses. The activation energy is the startup barrier that must be overcome for a reaction to get going: for an exergonic (or exothermic) reaction, the energy curve looks like
What happens to the kinetic energy of a fluid?
Increasing temperature increases the Kinetic Energy that molecules possess. In a fluid, this means that there are more random collisions between molecules per unit time.
Why does increasing substrate concentration increase the rate of reaction?
This is because more substrate molecules will be colliding with enzyme molecules, so more product will be formed.
What is the transition state of a non-enzyme catalyzed reaction?
For the non-enzyme catalyzed reaction, transition state theory can be used to show that the first order rate constant k1= kT/h where k is the Boltzman's constant, T is the Kelvin temperature, and h is Planck's constant. Hence, using Keq = [S‡]/ [S], equation 1 can be derived
Which binds the transition state more tightly than the substrate?
M binds the transition state more tightly than the substrate
What happens when an ester is hydrolyzed?
When an ester is hydrolyzed, the sp2 hybridized carbonyl carbon is converted to an sp3 hybridized center in the intermediate, with the carbonyl oxygen becoming an oxyanion. The transition state presumably looks more like this unstable intermediate (sp3, oxyanion).
What is transition state theory?
Transition state theory can be used to more clearly quantify the relationships described in the graphical analysis above. This analysis will use the equilibrium constant (in contrast to the last two chapters which used dissociation constants to characterize macromolecule, receptor, and enzyme binding to ligand).
What does a catalyst do?
Linus Pauling postulated long ago that the only thing that a catalyst must do is bind the transition state more tightly than the substrate. That this must be the case can be seen from the diagram below, which shows how S and S ∗ (the transition state) can react with E to form a complex which then proceeds to product, or can go to product in the absence of E. From this diagram, it should be evident that c − a = d − b, where a is the Δ G o for the binding of S to E, and b is the Δ G o for the binding of S* to E. For an enzyme to be a catalyst the activation energy for the reaction in the presence of E, d, must be less than in the absence of enzyme, c. Therefore c − d = a − b > 0. Since Δ G o = − R T ln K e q, K e q for binding of S ∗ to E is greater than for S binding to E.
What happens when you inject a mouse with a protein?
When injected into a mouse (after first being covalently attached to a carrier protein so the small molecule becomes "immunogencic"), the mouse makes a protein antibody which binds to the phosphonate. When the corresponding carboxylic acid ester is added to the antibody, it is cleaved with nominal kcat and Km values.
