Basicity and nucleophilicity affect each other in the same way: as basicity increases, so does nucleophilicity, and as basicity decreases, so does nucleophilicity. Is nucleophilicity directly proportional to basicity? Yes, in general, nucleophilicity and basicity are directly proportional. What is the meaning of nucleophilicity?
Full Answer
How is nucleophilicity related to basicity?
Within a period, nucleophilicity parallels basicity and decreases from left to right in the periodic table for elements in similarly structured species with the same charge. For example, hydroxide ion is a better nucleophile than fluoride ion. Nucleophilicity is decreased by hydrogen bonding of protic solvents such as alcohols.
What are the trends in nucleophilicity?
Some useful trends have been documented: For a given element, negatively charged species are more nucleophilic (and basic) than are equivalent neutral species. For a given period of the periodic table, nucleophilicity (and basicity) decreases on moving from left to right.
How does nucleophilicity change from left to right on the periodic table?
As basicity decreases from left to right on the periodic table, nucleophilicity also decreases. When it comes to nucleophilicity, do not assign this same rule when making comparisons between the halogens located in a column.
What determines the nucleophilicity of an atom?
Here size dictates the nucleophilicity. Electronegativity: Basicity and nucleophilicity are inversely related to electronegativity. While electronegativity is the measure of how tightly an electron is held by the atom, basicity measures how easily an electron pair can be donated.

What is the relationship between basicity and nucleophilicity?
We consider the term basicity for species that are Bronsted bases. Thus, basicity comes from the ability of a nucleophile to approach a hydrogen atom. Thus, bases are nucleophiles with the ability to abstract hydrogen. However, the nucleophilicity of a nucleophile comes from the ability to donate a pair of electrons.
What does basicity mean in biology?
The term basicity denotes the strength of a basic species. A higher nucleophilicity will always result in an increased rate for the reaction. Change in nucleophilicity can alter the basicity also. Some of the factors which affect the nucleophilicity and there by basicity are given below;
What is a negative nucleophile?
Negative nucleophiles: They carry a negative charge and have an excess pair of electrons. The electron pair can be donated to an electron deficient species in order to form a new bond. The product formed with a negatively charged nucleophile will be neutral in nature. Some examples of negative nucleophiles are:
Why do compounds have nucleophiles?
Compounds containing heteroatoms like oxygen, nitrogen, sulfur and so on can act as a nucleophile due to the presence of unshared pair of electrons.
Nucleophilicity and basicity Definition
Nucleophilicity and basicity have very close connections such that the nucleophiles are typically Lewis bases. Thus, the nucleophilicity of a nucleophile comes from the ability to donate a pair of electrons. However, we consider the term basicity for species that are Bronsted bases.
Overview of Nucleophilicity And Basicity
Almost all of the organic reactions involve the attack of a reagent on the substrate molecule and in this context, the terms nucleophilicity and basicity have its own importance. Nucleophilic reagents are electron rich species that tend to attack positive centers.
Factors affecting the nucleophilicity
The term nucleophilicity denotes the strength of a nucleophile species. A higher nucleophilicity will always result in an increased rate for the reaction. Some of the factors which affect the nucleophilicity are given below:
Types of nucleophiles
1. Negative nucleophiles: They carry a negative charge and have an excess pair of electrons. The electron pair can be donated to an electron deficient species in order to form a new bond. The product formed with a negatively charged nucleophile will be neutral in nature. Some examples of negative nucleophiles are:
How is nucleophilicity decreased?
Nucleophilicity is decreased by hydrogen bonding of protic solvents such as alcohols. Within a group, the order of nucleophilicity is opposite to the order of basicity. This order of nucleophilicity is related to the polarizability of the nucleophile.
What is nucleophilicity in chemistry?
Nucleophilicity refers to the ability of a nucleophile to displace a leaving group in a substitution reaction. We describe various trends in nucleophilicity in this section. There is a trend within a period, a trend within a group, a trend based on the charge of the nucleophile, and a steric effect of the nucleophile. Nucleophiles are also bases, and they can abstract protons in elimination reactions. However, although nucleophilicity and basicity are related to the availability of the same electron pair, the reactions of a series of nucleophiles do not necessarily parallel those of the same species as bases.
What is the nucleophilic reaction?
Nucleophilicity refers to the ability of a nucleophile to displace a leaving group in a substitution reaction. We will describe trends in nucleophilicity in Chapter 10. Most common nucleophiles have a negative charge. However, it is the nonbonding electron pair that is important.
What is the nucleophilicity of the N-donors and C-to-O migration of the?
The nucleophilicity of the N-donors and C-to-O migration of the trimethylsilyl group is evident in the reaction between 16 and 1 atm of CO gas at room temperature, which resulted in the rapid and quantitative formation of the remarkable triple carbene pincer complex [ (CO)Pt {η 3 -C (Ph 2 P=NCOSiMe 3) 2 }] ( 19, Scheme 14.5, Fig. 14.16) [ 91 ]. This transformation presumably occurred via initial displacement of the cod and twofold attack by free iminophosphorane nitrogen atoms on coordinated CO followed by trimethylsilyl group migration. This ultimately resulted in the insertion of CO into both N–Si bonds and the concomitant formation of two new (nominally Fischer) carbene donors.
What happens when a nucleophile has a negative charge?
If the nucleophile has a negative charge, and it reacts with a neutral substrate, then the leaving group also has a negative charge.
What is the basicity of ethylene oxide?
The low basicity of ethylene oxide is, at least partly, attributed to the ring strain. The angle of the unperturbed C O C bond is close to 110°, whereas in ethylene oxide it equals 60°. For tricoordinated oxygen having sp2 hybridization, the C O C angle is equal to 120°; thus protonation (as well as alkylation) of ethylene oxide leads to an increase of the ring strain.
How to make 2-chloroalkanoic acid?
2-Chloroalkanoic acids can be prepared with high ee by the diastereoselective halogenation 3 17 of sugar-derived silyl ketene acetals with N -chlorosuccinimide.
What is the definition of nucleophilicity?
Recall the definitions of electrophile and nucleophile: Electrophile: An electron deficient atom, ion or molecule that has an affinity for an electron pair, and will bond to a base or nucleophile.
What is a nucleophile?
Nucleophile: An atom, ion or molecule that has an electron pair that may be donated in forming a covalent bond to an electrophile (or Lewis acid). If we use a common alkyl halide, such as methyl bromide, and a common solvent, ethanol, we can examine the rate at which various nucleophiles substitute the methyl carbon.
How do nucleophilic anions solvate?
Polar, protic solvents such as water and alcohols solvate anions by hydrogen bonding interactions , as shown in the diagram below.
When moving across a row, does nucleophilicity follow basicity?
To say that nucleophilicity follows basicity across a row means that, as basicity increases from right to left on the periodic table, nucleophilicity also increases. As basicity decreases from left to right on the periodic table, nucleophilicity also decreases.
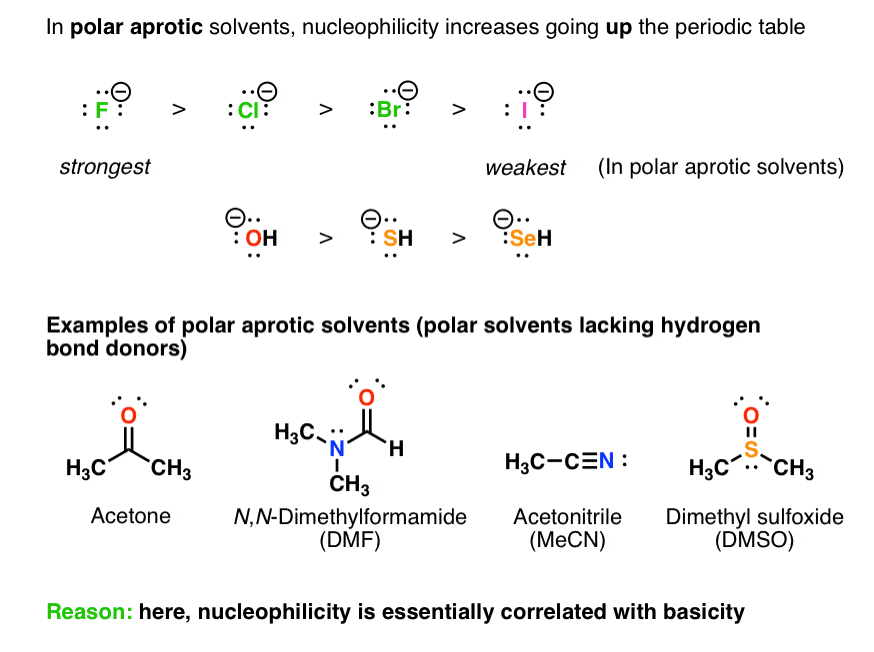
What are the consequences of the weak interaction between aprotic and nucleophiles?
A second consequence that results from the weak interaction that occurs between aprotic solvents and nucleophiles is that, under some conditions, there can be an inversion of the reactivity order. An inversion would result in nucleophilicity following basicity up and down a column, as shown in the following diagram. When we considered the effects of protic solvents, remember that the iodide anion was the strongest nucleophile. Now, in considering aprotic solvents under some conditions, the fluoride anion is the strongest nucelophile.
What is the result of a relatively weak interaction between the aprotic solvent and the nucleophile?
The result, with respect to solvation, is a relatively weak interaction between the aprotic solvent and the nucleophile. The consequence of this weakened interaction is two-fold. First, by using an aprotic solvent we can raise the reactivity of the nucleophile.
What are the factors that determine the nucleophilicity of a nucleophile?
Nucleophilicity depends on many factors, including charge, basicity, solvent, polarizability, and the nature of the substituents.
Which is better, nucleophile or reactive?
It has been experimentally shown that a nucleophile containing a negatively charged reactive atom is better than a nucleophile containing a reactive atom that is neutral. The next diagram illustrates this concept. Notice that when oxygen is part of the hydroxide ion, it bears a negative charge, and when it is part of a water molecule, it is neutral. The O of - OH is a better nucleophile than the O of H 2 O, and results in a faster reaction rate. Similarly, when nitrogen is part of NH 2, it bears a negative charge, and when it is part of NH 3, it is neutral. The N of NH 2 is a better nucleophile than the N of NH 3, and results in a faster reaction rate.
Can a nucleophile be neutral?
Nucleophiles can be neutral or negatively charged. In either case, it is important that the nucleophile be a good Lewis base, meaning it has electrons it wants to share. The following diagram is just a reminder of some of the nucleophiles that were presented in the section covering nucleophilic substitution.
Does nucleophilicity follow basicity?
In effect, when using protic solvents, nucleophilicity does not follow basicity when moving up and down a column. In fact, it's the exact opposite: when basicity decreases, nucleophilicity increases and when basicity increases, nucleophilicity decreases.
