What foods help increase the pH of blood?
The 14 Best Foods to Increase Blood Flow and Circulation
- Cayenne Pepper. Cayenne pepper gets its spicy flavor from a phytochemical called capsaicin. ...
- Pomegranate. Pomegranates are juicy, sweet fruits that are particularly high in polyphenol antioxidants and nitrates, which are potent vasodilators.
- Onions. ...
- Cinnamon. ...
- Garlic. ...
- Fatty Fish. ...
- Beets. ...
- Turmeric. ...
- Leafy Greens. ...
- Citrus Fruits. ...
How does the body maintain a constant blood pH?
the blood in the capillaries. The body has a wide array of mechanisms to maintain homeostasis in the blood and extracellular fluid. The most important way that the pH of the blood is kept relatively constant is by buffers dissolved in the blood. Other organs help enhance the homeostatic function of the buffers. For
What are five different ways the body maintains homeostasis?
Types of Homeostatic Regulation
- Thermoregulation. When you think about homeostasis, temperature might come to mind first. ...
- Osmoregulation. Osmoregulation strives to maintain the right amount of water and electrolytes inside and outside cells in the body. ...
- Chemical Regulation. Your body regulates other chemical mechanisms as well to keep systems in balance. ...
How does the blood maintain homeostasis?
What are two ways your body maintains homeostasis?
- Temperature. The body must maintain a relatively constant temperature. …
- Glucose. The body must regulate glucose levels to stay healthy. …
- Toxins. Toxins in the blood can disrupt the body’s homeostasis. …
- Blood Pressure. The body must maintain healthy levels of blood pressure. …
- pH.
How does pH affect homeostasis?
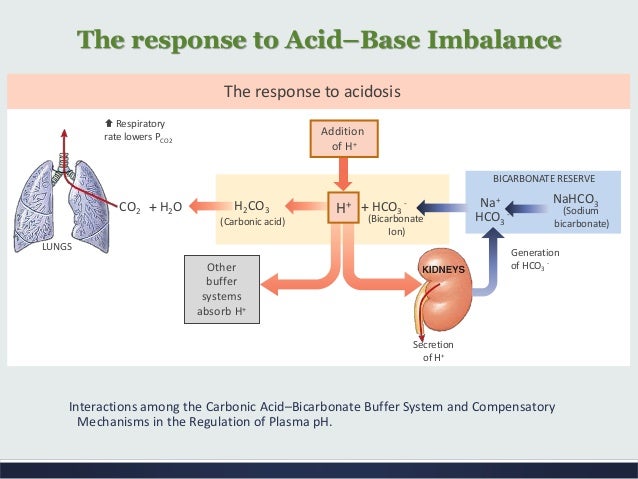
Regulation of body fluid pH is one of the most important physiological functions of homeostasis, because activity of most chemical reactions via enzyme proteins is dependent on fluid pH. To maintain homeostasis of body fluid pH, various buffering systems are utilized in addition to proton excretion from the cytosol to the extracellular space and ultimately outside of the body. However, if production of organic acid is elevated or the buffering and excretion systems are impaired, body fluid turns acidic, leading to abnormal conditions. A typical example is elevation of lactic acid production in skeletal muscle in response to strenuous exercise, which leads to body fluid acidosis, preventing muscle contraction [6, 7]. Proton transport across the plasma membrane of muscle cells is important for maintaining the appropriate intracellular pH. Skeletal muscle is a major metabolic organ that generates acids, in particular during contraction. Strenuous muscle contractions can cause a drastic reduction in intramuscular pH to −6.5 with accumulation of more than 40 mM lactate [6–8], regardless of cellular buffering capacity. Several studies have shown that intracellular pH is reduced during muscle contraction and has a delayed recovery to basal conditions during the recovery phase in the absence of proton transporters [9]. This delay suggests that proton transporters play a key role in maintaining pH homeostasis. Indeed, the function of proton transporters is involved in the capacity for pH maintenance [9, 10]. In particular, over 80% of intracellular proton is transported through lactate cotransport in contracting muscle, although remaining parts are transported through NHE and bicarbonate-depending transport [8, 11]. The liver, another organ that is closely associated with the metabolism of organic acids, generates ketone bodies (i.e., acetoacetic and β-hydroxybutyric acids), metabolizes lipids, and converts lactate to glucose via gluconeogenesis. Therefore, this organ generates acidic conditions [12–14] and intracellular pH should be maintained by proton extrusion along with buffering function.
How does the body maintain its pH?
Body fluid pH is strictly maintained by buffering systems, efflux across plasma membrane, and acid excretion. Monocarboxylate transporter (MCT) and Na+/H+exchanger (NHE) contribute to proton extrusion from the cytosol to the extracellular space. In contrast to intracellular fluid and blood containing pH buffers such as Hb (hemoglobin) and albumin, the interstitial fluid pH could be easily reduced by acid stress owing to the limited availability of the buffering factors such as proteins.
What is the role of protons in maintaining pH in cells?
In addition to buffering systems such as the bicarbonate-carbonate system, protein-proton binding, and phosphoric acid, several membrane transporters are responsible for proton removal from the cytosol and play important roles in maintaining the alkaline pH in cells (Figure 1). In most mammalian cells, H+-monocarboxylate cotransporters (MCTs) participate in the transport of monocarboxylic acids such as lactate, pyruvate, beta-hydroxybutyrate, and acetoacetate across the cellular membrane by cotransporting protons and monocarboxylate anions [1–3]. Other transporters such as the Na+/H+exchanger (NHE) and bicarbonate-dependent exchanger also contribute to proton extrusion from the cytosol to the extracellular space [4, 5]. This review focuses on the critical role of the membrane transport system of protons in regulation of intracellular and extracellular fluid pH and its importance in maintaining physiological homeostasis and preventing diseases development.
How do protons transport in the body?
If not, they are transported to the extracellular fluid through the plasma membrane and buffered in circulation or excreted in urine and expiration gas. Several transporters including monocarboxylate transporters and Na+/H+exchanger play an important role in uptake and output of protons across plasma membranes in cells of metabolic tissues including skeletal muscle and the liver. They also contribute to maintenance of the physiological pH of body fluid. Therefore, impairment of these transporters causes dysfunction of cells, diseases, and a decrease in physical performance associated with abnormal pH. Additionally, it is known that fluid pH in the interstitial space of metabolic tissues is easily changed due to little pH buffering capacitance in interstitial fluids and a reduction in the interstitial fluid pH may mediate the onset of insulin resistance unlike blood containing pH buffers such as Hb (hemoglobin) and albumin. In contrast, habitual exercise and dietary intervention regulate expression/activity of transporters and maintain body fluid pH, which could partly explain the positive effect of healthy lifestyle on disease prognosis.
How is pH determined?
Body fluid pH is determined by the content of protons (H+) generated from organic acids produced in living cells. Lactic acid (lactate−/H+) is a typical proton source and is involved in the regulation of physiological pH. In metabolic tissues such as skeletal muscle and adipose tissue, the glycolytic anaerobic metabolism mediates the conversion of glucose and glycogen into lactic acid. Because the pKa of lactic acid is 3.80, it is immediately dissociated into lactate (lactate−) and protons under physiological conditions, resulting in reduced intracellular pH. Pyruvic acid (pyruvate−/H+), an intermediate metabolite in the glycolytic system, is also a source of protons, although it generates much less protons compared to lactic acid. In addition, metabolites such as ketone bodies also act as proton sources. Beta-hydroxybutyric acid (beta-hydroxybutyrate−/H+), a typical ketone body, is generated as a result of fatty acid metabolism in the liver and is also dissociated into beta-hydroxybutyrate anions and protons, leading to the reduction of intracellular pH.
Why is membrane transport important?
Membrane transport of protons is required for preventing acidic states of body fluid, maintaining physical performance, and improving metabolic impairments. In contrast to the intracellular and blood pH, interstitial fluid pH can easily be reduced by acid stress.
Which organ is closely associated with the metabolism of organic acids?
The liver, another organ that is closely associated with the metabolism of organic acids, generates ketone bodies (i.e., acetoacetic and β-hydroxybutyric acids), metabolizes lipids, and converts lactate to glucose via gluconeogenesis.
How does homeostasis work?
Homeostasis is maintained by the respiratory system in two ways: gas exchange and regulation of blood pH. Gas exchange is performed by the lungs by eliminating carbon dioxide, a waste product given off by cellular respiration. As carbon dioxide exits the body, oxygen needed for cellular respiration enters the body through the lungs. ATP, produced by cellular respiration, provides the energy for the body to perform many functions, including nerve conduction and muscle contraction. Lack of oxygen affects brain function, sense of judgment, and a host of other problems.
Why do buffers release H+ ions?
On the other hand, the lack of H+ ions causes the blood to be too basic (alkalosis). In this situation, buffers release H+ ions. Buffers function to maintain the pH of our blood by either donating or grabbing H+ ions as necessary to keep the number of H+ ions floating around the blood at just the right amount.
What happens when blood enters the pulmonary capillaries?
When blood enters the pulmonary capillaries, the bicarbonate ions and hydrogen ions are converted to carbonic acid ( H 2 CO 3) and then back into carbon dioxide (CO 2) and water. This chemical reaction also uses up hydrogen ions. The removal of these ions gives the blood a more neutral pH, allowing hemoglobin to bind up more oxygen.
Why is oxygen able to bind to blood cells?
It is able to do this because the capillaries are permeable to oxygen. After it is in the capillary, about 5% will be dissolved in the blood plasma. The other oxygen will bind to red blood cells. The red blood cells contain hemoglobin that carries oxygen.
How does carbon dioxide get into the body?
Each body cell releases carbon dioxide into nearby capillaries by diffusion, because the level of carbon dioxide is higher in the body cells than in the blood.
How much more oxygen can a blood cell transport without hemoglobin?
Blood with hemoglobin is able to transport 26 times more oxygen than plasma without hemoglobin. Our bodies would have to work much harder pumping more blood to supply our cells with oxygen without the help of hemoglobin. Once it diffuses by osmosis it combines with the hemoglobin to form oxyhemoglobin.
Where does carbon dioxide go in the blood?
In the capillaries, some of the carbon dioxide is dissolved in plasma and some is taken by the hemoglobin, but most enters the red blood cells where it binds with water to form carbonic acid. It travels to the capillaries surrounding the lung where a water molecule leaves, causing it to turn back into carbon dioxide.
How does the lungs regulate blood pH?
The lungs can help regulate blood pH rapidly through the process of exhaling carbon dioxide, sometimes producing changes within seconds. For example, when someone exercises, they produce more carbon dioxide, so they breathe faster to prevent the blood from becoming too acidic.
What does the pH of blood mean?
The pH of blood refers to how acidic it is. Changes to blood pH can signal underlying medical issues. The pH scale, otherwise known as the acid-base scale, runs from 0 to 14. It measures how acidic a solution of a substance in water is. For example, pure water has a pH of 7. Solutions with a low pH have a high concentration ...
Why does respiratory alkalosis occur?
Respiratory alkalosis often occurs due to situations or conditions that make people breathe quicker or deeper than usual . These include:
What is metabolic acidosis?
Metabolic acidosis: This occurs due to reduced bicarbonate or increased acid levels.
How to find out if someone has acid or base?
Blood pH tests. There are two main types of tests that doctors can use to find out the pH of someone’s blood: arterial blood gas testing and electrolyte testing. Knowing the pH of a person’s blood can help a doctor find out if that person has an acid-base disorder.
What happens when pH changes?
When a change happens in one direction, there are mechanisms to move the acid-base balance the other way. For example, if a person has respiratory acidosis, there should be a metabolic response from the kidneys to reset the balance. If the body does not reset the pH balance, it can lead to more severe illness.
What does it mean when your pH is suddenly changing?
A sudden change in blood pH may indicate an underlying health problem. The pH of blood in the arteries should be between 7.35 and 7.45 for the body’s metabolic processes and other systems to work well. These processes produce acids, so the body has a complex system of feedback and regulation to maintain healthy pH levels.
