
How do you treat anemia of chronic disease?
chronic kidney disease (CKD) is a key factor fuelling demand for anemia treatment. Anemia treatment market is also receiving a strong impetus from the rising popularity of combination therapy. Increasing use of vitamin and iron supplements, antibiotics ...
What are the dangers of being anemic?
Anemia during pregnancy increases the chance of complications, such as:
- Premature birth
- Low birth weight babies
- Increased risk of abortions
- Puerperal sepsis (post-partum infections)
- Increased blood loss during delivery
- Increased risk of post-delivery depression
How can you tell if your anemic?
- Persistent fatigue, breathlessness, rapid heart rate, pale skin, or any other symptoms of anemia; seek emergency care for any trouble breathing or change in your heart beat.
- Poor diet or inadequate dietary intake of vitamins and minerals
- Very heavy menstrual periods
What are the most common symptoms of anemia?
- Weakness
- Shortness of breath
- Dizziness
- Fast or irregular heartbeat
- Pounding or "whooshing" in your ears
- Headache
- Cold hands or feet
- Pale or yellow skin
- Chest pain
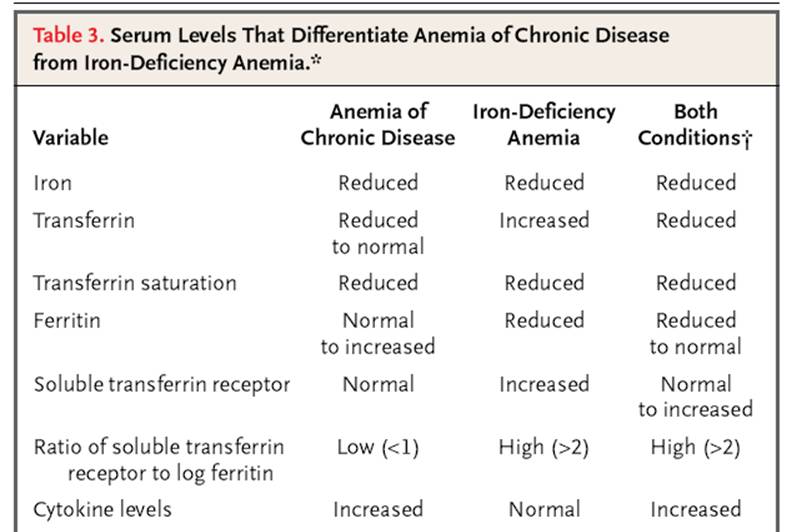
Why does chronic disease cause anemia?
Anemia of chronic disease happens when you have an autoimmune disease or other illness lasts longer than three months and that causes inflammation. Chronic inflammation can affect your body's ability to use iron needed to make enough red blood cells. Anemia happens when you don't have enough red blood cells.
Why does chronic inflammation cause Microcytic anemia?
In patients with inflammation, an abundance of hepcidin should lead to poor uptake of dietary iron from the gastrointestinal tract, iron sequestration in macrophages, little iron recycling to the erythron for red-cell production, and microcytic anemia. This pathophysiology is termed inflammatory block.
Why does inflammation decrease iron?
Inflammation associated with infections and inflammatory disorders would be expected to decrease iron absorption and reduce the efficacy of iron- fortified foods. The decreased absorption is due to an increase in circulating hepcidin in response to inflammatory cytokines.
What chronic disease causes anemia of chronic?
CausesAutoimmune disorders, such as Crohn disease, systemic lupus erythematosus, rheumatoid arthritis, and ulcerative colitis.Cancer, including lymphoma and Hodgkin disease.Long-term infections, such as bacterial endocarditis, osteomyelitis (bone infection), HIV/AIDS, lung abscess, hepatitis B or hepatitis C.
Why anemia of chronic disease is microcytic?
Worldwide, the anemia of chronic disease is the 2nd most common anemia. Early on, the red blood cells (RBCs) are normocytic; with time they may become microcytic. The major issue is that erythropoiesis is restricted due to inappropriate iron sequestration.
What type of anemia is anemia of inflammation?
Anemia of inflammation (AI, also called anemia of chronic disease) is a common, typically normocytic normochromic anemia that is caused by an underlying inflammatory disease. It is diagnosed when serum iron concentrations are low despite adequate iron stores, as evidenced by serum ferritin that is not low.
Does inflammation cause low ferritin?
Our study showed a positive correlation between ferritin and CRP, signifying an underlying low grade inflammation, leading to subsequent iron deficiency, most probably, because of inflammation mediated iron sequestration in the reticuloendothelial system.
How does inflammation affect ferritin levels?
Ferritin molecules help sequester this free iron, preventing its participation in this reaction and subsequent free radical-mediated cellular damage. Beyond this protective role in redox biology and iron homeostasis, free serum ferritin is increased in the setting of ongoing inflammation.
Why does transferrin decrease in inflammation?
As mentioned above, transferrin is a negative acute phase protein. When the liver increases the production of inflammation-associated proteins (e.g. CRP, ferritin) it decreases the production of transferrin. A number of conditions such as infection and cancer can decrease transferrin levels [6, 7, 28].
What are the 3 main causes of anemia?
Hemoglobin is an iron-rich protein that gives the red color to blood. It carries oxygen from the lungs to the rest of the body. Anemia has three main causes: blood loss, lack of red blood cell production, and high rates of red blood cell destruction.
Does anemia cause inflammation in the body?
While anemia of inflammation typically develops slowly, anemia of critical illness is a type of anemia of inflammation that develops quickly in patients who are hospitalized for severe acute infections, trauma, or other conditions that cause inflammation.
What autoimmune diseases can cause anemia?
What disorders can cause autoimmune hemolytic anemia?Lupus.Rheumatoid arthritis.Sjogren's syndrome.Thyroid disease.Ulcerative colitis.Hashimoto's disease.
Is anemia of chronic disease normocytic or microcytic?
Anemia of chronic disease is the most common normocytic anemia and the second most common form of anemia worldwide (after iron deficiency anemia). The MCV may be low in some patients with this type of anemia.
Can anemia of chronic disease be Macrocytic?
Both iron deficiency anemia and anemia of chronic disease can manifest with normocytic anemia in the initial phase and microcytic anemia later on. Bone marrow failure (e.g., due to myeloproliferative malignancy, myelodysplastic syndrome) can manifest with microcytic, normocytic, or macrocytic anemia.
What are three causes of microcytic anemia?
The major causes of microcytic anemia in adults are iron deficiency, inflammatory disease, and thalassemia. The most common cause of microcytic anemia is iron deficiency.
What is the most common cause of microcytic anemia?
Iron deficiency is the most common cause of microcytic anemia. The absence of iron stores in the bone marrow remains the most definitive test for differentiating iron deficiency from the other microcytic states, ie, anemia of chronic disease, thalassemia, and sideroblastic anemia.
What is anemia of chronic disease?
Anemia of inflammation (AI, also called anemia of chronic disease) is a common, typically normocytic normochromic anemia that is caused by an underlying inflammatory disease . It is diagnosed when serum iron concentrations are low despite adequate iron stores, as evidenced by serum ferritin that is not low. In the setting of inflammation, AI may be difficult to differentiate from iron deficiency anemia, and the two conditions may coexist. In AI, erythropoiesis is iron-restricted by hepcidin-mediated hypoferremia and erythrocyte production is suppressed by cytokines acting on erythroid progenitors. Decreased erythropoiesis is unable to compensate for shortened erythrocyte lifespan caused by enhanced erythrophagocytosis by cytokine-activated macrophages. Treatment should focus on the underlying disease. If this is not feasible and the anemia limits the quality of life or the performance of daily activities, a combination of erythropoiesis-stimulating agents and intravenous iron may be effective but should be attempted only after careful consideration of risk and benefit. Recent advances in molecular understanding of AI are stimulating the development of new pathophysiologically targeted experimental therapies.
What is the lagging indicator of anemia?
The defining biochemical features of AI include low serum iron despite adequate systemic iron stores. The concentration of serum transferrin is also decreased during chronic inflammation but this is a lagging indicator because of the long half-life of transferrin (about 8 days) compared to iron (about 1.5 hours)2. The erythrocytes are usually of normal size and have normal hemoglobin content but are reduced in number (normocytic, normochromic anemia). In some cases, particularly if the inflammatory disease is longstanding, the red cells can be mildly decreased in size and hemoglobin content.
What is the pathogenesis of AI?
The pathogenesis of AI is mediated by inflammatory cytokines and hepcidin, acting together to suppress erythropoiesis and shorten erythrocyte survival in blood. The effects of cytokines are denoted in light green, hepcidin effects are depicted in orange, and combined effects in red.
What is the pathophysiology of AI?
Despite more than fifty years of investigation, our understanding of the pathophysiology of AI is incomplete. Already the earliest studies of AI indicated that the disorder is a consequence of a mild decrease in erythrocyte survival combined with impaired production of erythrocytes1;20. The increased destruction of erythrocytes is predominantly attributable to macrophage activation by inflammatory cytokines but other hemolytic mechanisms may contribute in specific inflammatory diseases. The suppression of erythrocyte production has two major components, iron restriction and direct cytokine effects on erythropoietic progenitors. These effects combine to limit the erythropoietic response to erythropoietin which becomes insufficient to compensate for the increased destruction of erythrocytes. In some situations, the production of erythropoietin may also be decreased, perhaps due to cytokine effects on the renal cells that produce the hormone. In severe inflammation, or when the primary pathology involves the kidneys, decreased renal excretion of hepcidin contributes to hepcidin accumulation and iron restriction21. The complex pathogenesis of AI is summarized in Figure 1and discussed further.
What cytokines suppress erythropoiesis?
Inflammatory cytokines , including TNFα, IL-1 and interferon-γ, have been reported to suppress erythropoiesis in vitro 37-41as well as in mouse models24;42. Detailed understanding of the mechanisms involved has been hindered by the complexity of cytokine effects and the ability of each cytokine to regulate the production of many other cytokines41. Nevertheless, several new and promising concepts about the effects of cytokines on erythropoiesis have recently emerged. Libregts at al24developed a mouse model where overproduction of interferon-γ leads to the development of a mild-to-moderate normocytic normochromic anemia. The model manifests a 50% decrease in erythrocyte survival attributable to interferon-γ-mediated activation of macrophages in the splenic red pulp. The model also shows suppression of erythrocyte production affecting the erythroblast stages and the earliest erythroid-committed precursor BFU-e (burst-forming unit-erythrocyte) but not proerythroblasts and CFU-e (colony-forming unit-erythrocyte). Importantly, myeloid CFU-G/M (colony-forming unit-granulocyte/macrophage) colonies were increased. Microarray analysis of erythroblasts indicated that interferon-γ promotes the transcription of PU.1 and its target genes in an IRF-1-dependent manner but does not affect GATA-1 or its targets. PU.1 and GATA-1 antagonize each other's activity so the increase in PU.1 would be expected to promote myelopoiesis at the expense of erythropoiesis. During infections with viruses or intracellular pathogens known to induce interferon-γ, this mechanism may assure sufficient production of monocytes and macrophages, at the expense of temporary impairment of erythropoiesis. Whether other inflammatory cytokines utilize a similar or different mechanism remains to be determined.
Is AI a secondary manifestation of inflammatory disorders?
AI is a secondary manifestation of inflammatory disorders, and treating the underlying disease will correct the anemia. Such treatment is not always possible. Direct treatment of anemia should be considered only if it is impairing the patient's performance, quality of life or recovery from underlying illness. Inflammatory diseases sufficiently severe to cause AI may also cause fatigue or malaise through cytokine-dependent mechanisms, so these symptoms need not be caused by anemia. Potential therapies for AI include erythrocyte transfusions usually reserved for severe and acutely symptomatic anemia, and erythropoiesis-stimulating agents (ESAs: erythropoietin and its derivatives, mimics or inducers, as they become available) with or without intravenous iron supplementation. AI is not a specifically-approved indication for the use of ESAs but should be considered as an alternative to chronic erythrocyte transfusion. The use of ESAs in AI is based on a small number of anecdotal reports49-53that reported improvement of anemia, and similarities between AI and anemia of chronic kidney disease (CKD), the main indication for ESAs. In CKD, IV iron supplementation potentiates the effect of erythropoietin and its derivatives54, and it has been reported that IV iron may have a similar activity in AI53.
Can anemia be treated if severe?
Treat anemia specifically only if severe or limits activities of daily living
What is the diagnosis of anemia?
Anemia, defined by a Hb concentration <120 g/L for women and <130 g/L for men, can be diagnosed as AI or ACD based on the underlying alterations in iron homeostasis along with clinical or biochemical evidence of inflammation; however, it is often necessary to rule out coexisting causes of anemia that may require specific interventions ( Table 2 ). In addition to assessing coexisting iron deficiency, as discussed further below, laboratory evaluations may include renal and liver function tests, thyroid function tests or markers of hemolysis, and determination of folic acid, cobalamin, or vitamin D concentrations. Vitamin D is a negative regulator of hepcidin expression. 54 Elderly subjects with different causes of anemia were often found to suffer from vitamin D deficiency, and anemia can be corrected, in part, by vitamin D supplementation, which decreases hepcidin levels. 54, 55
How long does it take for anemia to show up in a patient with severe infection?
View Large. Finally, in an acute clinical setting, anemia is detected after hours or a few days in patients with severe infection, which cannot be solely explained by inflammation-driven iron retention or inhibition of erythropoiesis, which may require more time to result in a clinically evident reduction in Hb.
What are the mechanisms of AI?
Systemic inflammation results in immune cell activation and formation of numerous cytokines. Interleukin (IL-6) and IL-1β, as well as lipopolysaccharide (LPS), are potent inducers of the master regulator of iron homeostasis, hepcidin, in the liver, whereas expression of the iron-transport protein transferrin is reduced. Hepcidin causes iron retention in macrophages by degrading the only known cellular iron exporter ferroportin (FP1); by the same mechanism, it blocks dietary iron absorption in the duodenum. Multiple cytokines (eg, interleukin-1β [IL-1β], IL-6, IL-10, and interferon-γ [IFN-γ]) promote iron uptake into macrophages, increase radical-mediated damage to erythrocytes and their ingestion by macrophages, and cause efficient iron storage by stimulating ferritin production and blocking iron export by transcriptional inhibition of FP1 expression. This results in the typical changes of AI (ie, hypoferremia and hyperferritinemia). In addition, IL-1 and TNF inhibit the formation of the red cell hormone erythropoietin (Epo) by kidney epithelial cells. Epo stimulates erythroid progenitor cell proliferation and differentiation, but the expression of its erythroid receptor (EpoR) and EpoR-mediated signaling are inhibited by several cytokines. Moreover, cytokines can directly damage erythroid progenitors or inhibit heme biosynthesis via radical formation or induction of apoptotic processes. Importantly, because of iron restriction in macrophages, the availability of this metal for erythroid progenitors is reduced. Erythroid progenitors acquire iron mainly via transferrin-iron/transferrin receptor (Tf/TfR)-mediated endocytosis. Erythroid iron deficiency limits heme and hemoglobin (Hb) biosynthesis, as well as reduces EpoR expression and signaling via blunted expression of Scribble (Scb). In addition, the reduced Epo/EpoR signaling activity impairs the induction of erythroferrone (Erfe), which normally inhibits hepcidin production.
What are the pathogenic factors of AI?
The second pathogenic factor in AI is iron- and hepcidin-independent impairment of erythropoiesis. It is caused, in part, by reduced production and/or reduced biological activity of the hormone Epo in the inflammatory setting. 34 Observational studies have indicated lower Epo levels than expected for the degree of anemia in most AI subjects. 1 These observations may be due, in part, to the inhibitory effects of cytokines, such as IL-1 and TNF, on hypoxia-mediated stimulation of Epo by interfering with mediated GATA-2 or HNF4 transcription or by causing radical-mediated damage of Epo-producing kidney epithelial cells. 35, 36 Epo exerts its biological effects after binding to its homodimeric erythroid receptor via JAK/STAT-mediated signaling cascades. 36 Although, the number of Epo receptors (EpoRs) did not appear to be altered in subjects with inflammatory anemia, the efficacy of Epo-mediated signaling is reduced and inversely linked to the circulating levels of IL-1 and IL-6, 37 indicating the inflammation-driven hyporesponsiveness of EpoRs. However, recent evidence suggests that erythroid iron deficiency, because it also occurs in AI, results in downregulation of EpoR, which could be traced back to iron-mediated regulation of the EpoR control element Scribble. 31 Specifically, iron deficiency impairs transferrin receptor-2–mediated iron sensing in erythroid cells, 38 resulting in downregulation of Scribble and reduced EpoR expression.
What cytokines affect iron absorption?
In addition, various cytokines directly impact on duodenal or macrophage iron homeostasis. Tumor necrosis factor (TNF) reduces duodenal iron absorption by a poorly characterized, but hepcidin-independent, mechanism. 20 The cytokines IL-1, IL-6, IL-10, or TNF-α promote iron acquisition into macrophages via transferrin receptor–mediated endocytosis, via divalent metal transporter 1, or possibly also via increased iron acquisition by lactoferrin and lipocalin-2. 21 However, the major source for iron for macrophages is senescent erythrocytes. Cytokines, inflammation-derived radicals, and complement factors damage erythrocytes and promote erythrophagocytosis via stimulation of receptors recognizing senescent red blood cells, such as T-cell immunoglobulin domain 4 or possibly CD44. 22, 23 Recent evidence suggests that, during periods of increased erythrocyte destruction, erythrophagocytosis and iron recycling are primarily carried out by hepatic macrophages differentiating in the liver from circulating monocytes, rather than by resident splenic macrophages. 24 Once iron is acquired by macrophages, it is mainly stored within ferritin, the major iron storage protein whose expression is massively induced by macrophage iron, heme, and cytokines. 5, 25 Although circulating and, to a lesser extent, macrophage-derived hepcidin are the main regulators of iron export by these cells, 15, 16, 18, 19, 26, 27 bacterial lipopolysaccharides and interferon-γ (IFN-γ) block the transcription of ferroportin, thereby reducing cellular iron export. 28, 29 All of these events lead to iron-restricted erythropoiesis and the characteristic changes in systemic iron homeostasis observed in AI: hypoferremia and hyperferritinemia. These effects are partially counteracted by the stimulation of synthesis of ferroportin in macrophages by retained iron and heme, 30 perhaps explaining why AI rarely reaches the severity seen in pure iron-deficiency anemia.
Why do erythrocytes have a shorter lifespan?
A shortened erythrocyte lifespan has been extensively documented in the inflammatory setting and has been attributed to enhanced erythrophagocytosis by hepatic and splenic macrophages caused by “bystander” deposition of antibody and complement on erythrocytes, mechanical damage from fibrin deposition in microvasculature , and activation of macrophages for increased erythrophagocytosis. 23, 46, 47 Shortened erythrocyte survival is usually a minor factor in chronic AI; however, in acute infections, severe sepsis, or other critical illnesses accompanied by a high level of cytokine activation, anemia is detected after hours or a few days (ie, too rapidly to be accounted for by deficient erythropoiesis). It is reasonable that massive erythrophagocytosis, hemolysis, or pooling of erythrocytes, along with hemodilution, contribute to this entity that awaits systematic scientific analysis. 48 Moreover, remediable iatrogenic factors are common in critical illness and include blood loss from phlebotomy and gastrointestinal blood loss caused by nasogastric tubes, anticoagulation, and the use of medications that promote gastroduodenal erosion or ulceration.
What is the most common anemia in hospitalized patients?
Anemia of inflammation ( AI), also known as anemia of chronic disease ( ACD), is regarded as the most frequent anemia in hospitalized and chronically ill patients. It is prevalent in patients with diseases that cause prolonged immune activation, including infection, autoimmune diseases, and cancer. More recently, the list has grown ...
What are the causes of anemia?
There are three primary causes of anemia: blood loss , lack of red blood cell production , and high rates of red blood cell destruction.
What is the best treatment for chronic anemia?
For example, if you have IBD, your doctor might prescribe anti-inflammatories such as corticosteroids and antibiotics such as ciprofloxacin ( Cipro ). These can treat the IBD and make the chronic anemia disappear.
What supplements are prescribed for chronic anemia?
For example, if you have kidney disease with chronic anemia, your doctor might prescribe vitamin B-12 and folic acid supplements if you have a vitamin B-12 or folate deficiency. Or your doctor might prescribe a synthetic form ...
What causes red blood cells to be rigid and clog circulation through small blood vessels?
Sickle cell anemia. Sickle cell anemia is an inherited hemolytic anemia with abnormal hemoglobin protein that causes red blood cells to be rigid and clog circulation through small blood vessels.
What is the most common type of anemia?
Iron deficiency anemia is the most common type of anemia. It’s caused by a lack of iron from blood loss, a diet deficient in iron, or poor absorption of iron.
What is it called when your bone marrow stops making enough blood cells?
Aplastic anemia is a rare condition that occurs when your bone marrow stops making enough blood cells.
Why is my blood not getting enough oxygen?
If you have anemia, you have a lower-than-normal number of red blood cells, or the amount of hemoglobin in your red blood cells has dropped below normal. Because of this, your body’s cells aren’t getting enough oxygen. There are three primary causes of anemia: blood loss, lack of red blood cell production, and high rates ...
Why do people with chronic kidney disease get anemia?
Chronic kidney disease (Nearly every patient with this type of disease will be get anemia because kidneys make erythropoietin (EPO), a hormone that controls the production of red blood cells in the bone marrow.)
What are the symptoms of anemia?
Symptoms are similar to those of iron-deficiency anemia and include fatigue, sweating, and headaches. Overview. Symptoms and Causes. Diagnosis and Tests. Management and Treatment. Prevention. Outlook / Prognosis. Anemia of Chronic Disease.
Why do red blood cells die?
Chronic diseases may cause changes in red blood cells, the oxygen-carrying blood cells made by bone marrow. These changes can cause red blood cells to die sooner and slow down their production. In anemia of chronic disease, the iron that is normally recycled from old red blood cells to help make new red blood cells is retained within a system ...
How long does anemia last?
Chronic diseases are those that last longer than 3 months.
Why are blood transfusions not used as a long term treatment?
Transfusions are not used as a long-term therapy because of risks—such as iron overload and potential immune system side effects— that may increase the risk of getting an infection.
What is the most common inflammatory disease that affects the lung and lymph glands?
Sarcoidosis, an inflammatory disease that commonly affects the lung and lymph glands, most likely caused by an abnormal immune response. Inflammatory bowel disease ( Crohn’s disease and ulcerative colitis ), which affects the intestines. Chronic rejection of a transplanted organ. Heart failure.
Can anemia mask symptoms?
The symptoms of the disease causing the anemia may mask the symptoms of the anemia itself , so doctors will want to perform a blood test.
What causes anemia of inflammation?
Although the exact cause of anemia of inflammation is not known, it is related to the effects of chronic inflammatory diseases on the red blood cells. These conditions cause a number of changes in the body's red blood cells. The lifespan of red blood cells becomes shorter, production of new red blood cells in the bone marrow slows down, and iron is "withheld" so that it cannot be used to make new red blood cells. Normally the body recycles iron from "old" red blood cells and uses it to make new ones. In anemia of inflammation, the body does not recycle iron as easily, so it is "held up" in cells such as macrophages (a type of white blood cell). There is also decreased iron absorption from the intestines. These changes are caused by a protein called hepcidin.
Why does anemia of inflammation go unnoticed?
Anemia of inflammation may go unnoticed and untreated because the attention is centered on the disease that is causing it. In the past, it was believed that anemia of inflammation was associated only with infections such as syphilis and tuberculosis.
What does it mean when your TIBC is low?
A low TIBC and a normal or elevated ferritin level are the most important signs that indicate that anemia of inflammation is present. With inflammation, the level of certain plasma proteins called acute phase proteins is higher in the blood. The increase in these proteins usually leads to an increase in the blood's sedimentation rate, which is determined by a blood test.
What is anemia in health?
Anemia occurs when there aren't enough healthy red blood cells in the blood. Most anemias are more of a symptom than a disease and can be the result of a variety of health conditions. With any form of anemia, it's important to find the cause before treatment begins. Anemia of inflammation, also known as anemia of chronic disease, ...
What are the signs of inflammation?
Accurate diagnosis depends on blood test results such as the following: A low TIBC and a normal or elevated ferritin level are the most important signs that indicate that anemia of inflammation is present. With inflammation, the level of certain plasma proteins called acute phase proteins is higher in the blood.
What are some examples of anemia?
The following are examples of conditions that can cause anemia of inflammation: autoimmune diseases or diseases with inflammation (e.g., rheumatoid arthritis, lupus, ulcerative colitis, Crohn's disease, giant cell [temporal] arteritis)
Why is it important to treat anemia?
Once all other causes of anemia are ruled out and the inflammation, infection, or other problem is identified and treated, the anemia may improve.
What is the condition where the amount of hemoglobin in the blood drops below normal?
#TAB# Anemia is a condition in which a person has a lower than normal number of red blood cells or the amount of hemoglobin in the red blood cells drops below normal, which prevents the body’s cells from getting enough oxygen.
What is the National Institute of Diabetes and Digestive and Kidney Diseases?
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), through its Division of Kidney, Urologic, and Hematologic Diseases, conducts and supports research on anemia and other blood diseases . The NIDDK also conducts and supports basic research on the regulation of iron absorption, storage, and utilization, and on some of the chronic diseases associated with AI/ACD. For example, the NIDDK’s End-stage Renal Disease Program promotes research aimed at reducing medical problems stemming from blood, bone, nervous system, metabolic, GI, cardiovascular, and endocrine abnormalities in end-stage kidney failure.
Can anemia cause no symptoms?
Anemia of inflammation and chronic disease typically develops slowly and, because it is usually mild, may cause few or no symptoms . Symptoms of anemia may also be masked by the symptoms of the underlying disease. Sometimes, AI/ACD can cause or contribute to
