What is the rate of SN1 reaction?
However: SN1 reactions are unimolecular: the rate of this reaction depends only on the concentration of one reactant. SN1 reactions happen in two steps: The leaving group leaves, and the substrate forms a carbocation intermediate. The nucleophile attacks the carbocation, forming the product.
How does concentration affect SN2 reactions?
How does concentration affect SN2 reactions? Increasing the concentration of either the nucleophile or the substrate increases the reaction rate. In an SN2 reaction, one bond is broken and another bond is formed at the same time. Consider the reaction of hydroxide ion with chloromethane.
What is the difference between Sn2 and SN1?
SN1 reactions are nucleophilic substitutions, involving a nucleophile replacing a leaving group (just like SN2). However: SN1 reactions are unimolecular: the rate of this reaction depends only on the concentration of one reactant.
What is the solvent used in SN1 reaction?
Protic solvent such as water or alcohol are used in SN1 reactions since they solvate and stabilize the intermediate carbocation. The nucleophile is also solvated, but this has no effect on the reaction rate since the rate is dependent on the concentration of the alkyl halide.
Does SN1 depend on concentration?
The Rate Law Of The SN1 Reaction Is First-Order Overall When we do so, we notice that the rate is only dependent on the concentration of the substrate, but not on the concentration of nucleophile.
How does concentration affect SN2 reactions?
The rate of an SN2 reaction is first order in the substrate and first order in the nucleophile. If the substrate concentration is doubled, the reaction rate doubles. Similarly, if the concentration of the nucleophile is doubled, the rate again doubles.
How would doubling the concentration of the nucleophile affect the rate of an SN1 reaction?
The rate equation of an SN1 reaction is: k [R-X]. Notice that the nucleophile is not in the rate equation. Therefore, doubling the concentration of the nucleophile will have no effect on the rate of the reaction.
Does SN1 depend on concentration of nucleophile?
Sn1 Reaction doesnot depend on concentration of the nucleophile , so more the concentration of nucleophile, the relative rate of Sn1 product diminishes. Hence ,when the competing reaction is a Sn2 pathway, lesser concentration of nucleophile would favour the Sn1 pathway.
What does SN1 rate depends on?
SN1 reactions are nucleophilic substitutions, involving a nucleophile replacing a leaving group (just like SN2). However: SN1 reactions are unimolecular: the rate of this reaction depends only on the concentration of one reactant.
What are the factors affecting SN1 and SN2 reactions?
Factors affecting SN1 and SN2 reactionsNature of substrate.The nucleophilicity of the reagents.Solvent polarity.
What happens to the rate of an SN1 reaction when the concentration of the substrate is tripled and the concentration of the nucleophile is doubled?
What happens to the rate of a SN1 reaction when the concentration of the alkyl halide is tripled and the concentration of the nucleophile stays the same? The reaction rate is tripled.
Which of the following would increase the rate of an SN1 reaction pathway?
1 Answer. Increase the concentration of the alkyl halide.
How would increasing the concentration of the nucleophile affect the rate of each reaction?
1 Answer. Increasing the concentration of either the nucleophile or the substrate increases the reaction rate. In an SN2 reaction, one bond is broken and another bond is formed at the same time.
What conditions favor SN1 reactions?
In general, in order for an SN1 or E1 reaction to occur, the relevant carbocation intermediate must be relatively stable. Strong nucleophiles favor substitution, and strong bases, especially strong hindered bases (such as tert-butoxide) favor elimination.
What drives an SN1 reaction?
Greater nucleophilicity of the attacking group than the nucleophilicity of the leaving group causes SN1 reactions. The cleavage of the C-X bond keeps occuring always, both the reactant and the product remaining in the solution. The equilibrium shifts towards the final product.
Why do SN1 reactions prefer tertiary?
Formation of a planar carbocation in the first stage of the SN1 mechanism is favored for tertiary alkyl halides since it relieves the steric strain in the crowded tetrahedral alkyl halide. The carbocation is also more accessible to an incoming nucleophile.
What does the rate of an SN2 reaction depend on?
The bimolecular nucleophilic substitution reaction follows second-order kinetics; that is, the rate of the reaction depends on the concentration of two first-order reactants. In the case of bimolecular nucleophilic substitution, these two reactants are the haloalkane and the nucleophile.
What happens to the rate of an SN2 reaction when the concentration of the substrate is doubled and the concentration of the nucleophile is tripled?
What happens to the rate of a SN2 reaction when the concentration of the alkyl halide is halved and the concentration of the nucleophile is doubled? The reaction rate does not change.
How would increasing the concentration of the nucleophile affect the rate of each reaction?
1 Answer. Increasing the concentration of either the nucleophile or the substrate increases the reaction rate. In an SN2 reaction, one bond is broken and another bond is formed at the same time.
Which factor decreases the rate of SN2 reaction?
1) Steric bulk of the nucleophile – for similar species (e.g. alkoxide anions) the rate of substitution diminishes with an increased size of the nucleophile. 2) Steric effects in the substrate – the more substituted the carbon center is, the lower the rate of substitution.
What is an SN1 reaction?
SN1 stands for substitution nucleophilic unimolecular. The SN1 reaction is a nucleophilic substitution reaction where the rate-determining step is...
How many steps are there in the SN1 reaction?
SN1 reaction takes place in two steps. In the first step leaving group leaves and the substrate forms a carbocation intermediate. While in the seco...
What do SN1 reactions depend on?
SN1 reactions depend on one reactant’s concentration and are independent of the nucleophile’s strength.
What solvent is used in the SN1 reaction?
A polar protic solvent is used in the SN1 reaction as it stabilises the carbocation intermediate.
Why does SN1 favour weak nucleophiles?
A nucleophile is not involved in the rate-determining step. Thus, it is independent of the strength of the nucleophile.
What is an SN1 Reaction?
The S N 1 reaction is a nucleophilic substitution reaction where the rate determining step is unimolecular. It is a type of organic substitution reaction. S N 1 stands for substitution nucleophilic unimolecular. Thus, the rate equation (which states that the S N 1 reaction is dependent on the electrophile but not on the nucleophile) holds in situations where the amount of the nucleophile is far greater than the amount of the carbocation intermediate.
Which step of the SN1 reaction is the carbocation attacked by?
In the second step of the SN1 reaction mechanism, the carbocation is attacked by the nucleophile.
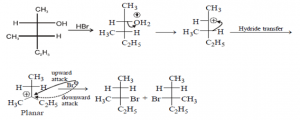
What happens to the carbocation reaction when it takes place at a stereocenter?
If the reaction takes place at a stereocenter and if neither avenue for nucleophilic attack is preferred, the carbocation is then attacked equally from both sides , yielding an equal ratio of left and right-handed enantiomers as shown below.
What is the carbocation intermediate formed in step 1 of the S N 1 reaction mechanism?
The carbocation intermediate formed in step 1 of the S N 1 reaction mechanism is an sp2 hybridized carbon. Its molecular geometry is trigonal planar, therefore allowing for two different points of nucleophilic attack, left and right.
When the bromide ion leaves the tertiary butyl bromide, a?
When the bromide ion leaves the tertiary butyl bromide, a carbocation intermediate is formed.
Can alkyl halides react with alcohol?
Thus, the tertiary/secondary alkyl halides can react with tertiary/secondary alcohols to undergo a nucleophilic substitution reaction. The halide is replaced with the nucleophile in the product.
Is the rate equation dependent on the nucleophile?
Thus, the rate equation (which states that the S N 1 reaction is dependent on the electrophile but not on the nucleophile) holds in situations where the amount of the nucleophile is far greater than the amount of the carbocation intermediate. This reaction involves the formation of a carbocation intermediate.
What happens to the reaction rate when you increase the concentration of a reactant?
If you increase the concentration of any reactant, the reaction rate will increase.
What increases the rate of a reaction?
Increasing the concentration of either the nucleophile or the substrate increases the reaction rate.
What determines whether substitution will be SN1 or SN2?
These include the nature of the nucleophile and the type of solvent used. The reactivity of primary, secondary, and tertiary alkyl halides is controlled by electronic and steric factors.
What are the factors that affect the rate at which alkyl halides undergo the SN2 reaction?
There are two factors which affect the rate at which alkyl halides undergo the SN2 reaction – electronic and steric. In order to illustrate why different alkyl halides react at different rates in the SN2 reaction, we shall compare a primary, secondary, and tertiary alkyl halide (Fig. 1).
What is the mechanism of substitution of alkyl halides?
There are two different mechanisms involved in the nucleophilic substitution of alkyl halides. When polar aprotic solvents are used, the SN2 mechanism is preferred. Primary alkyl halides react more quickly than secondary alkyl halides, with tertiary alkyl halides hardly reacting at all. Under protic solvent conditions with nonbasic nucleophiles (e.g. dissolving the alkyl halide in water or alcohol), the SN1 mechanism is preferred and the order of reactivity is reversed. Tertiary alkyl halides are more reactive than secondary alkyl halides, and primary alkyl halides do not react at all.
What does it mean when the reaction rate is affected by the concentration of the alkyl halide and the nu?
Measuring how the reaction rate is affected by the concentration of the alkyl halide and the nucleophile determines whether a nucleophilic substitution is SN2 or SN1. Measuring the optical activity of products from the nucleo-philic substitution of asymmetric alkyl halides indicates the type of mecha-nism involved. A pure enantiomeric product indicates an SN2 reaction. A partially or fully racemized product indicates an SN1 reaction.
What solvent is used in SN1?
Protic solvent such as water or alcohol are used in SN1 reactions since they solvate and stabilize the intermediate carbocation. The nucleophile is also solvated, but this has no effect on the reaction rate since the rate is dependent on the concentration of the alkyl halide.
Why is a planar carbocation favored in the first stage of the SN1 mechanism?
Formation of a planar carbocation in the first stage of the SN1 mechanism is favored for tertiary alkyl halides since it relieves the steric strain in the crowded tetrahedral alkyl halide. The carbocation is also more accessible to an incoming nucleophile. The formation of the carbocation is helped by electronic factors involving the inductive and hyperconjugationeffects of the three neighboring alkyl groups. Such inductive and hyperconjugation effects are greater in carbocations formed from tertiary alkyl halides than from those formed from primary or secondary alkyl halides.
Which is more likely to produce a stable intermediate than a primary or secondary alkyl halide?
Therefore, tertiary alkyl halides are far more likely to produce a stable carbocation intermediate than primary or secondary alkyl halides. It is important to realize that the reaction rate is determined by how well the transition state of the rate determining step is stabilized. In a situation like this where a high energy intermediate is formed (i.e. the carbocation), the transition state leading to it will be closer in character to the intermediate than the starting material. Therefore, any factor which stabilizes the intermediate carbocation also stabilizes the transition state and consequently increases the reaction rate.
What are the factors that increase the electron density of carbon atoms?
Carbocations are electron deficient species and the factors which can increase the electron density on the carbon atom can stabilize the carbocation. This includes the nature of the alkyl group, inductive effect, hyperconjugation, aromaticity and resonance. As we look from methyl to primary, secondary and tertiary alkyl groups, the stability of the corresponding carbocation formed is increasing.
Does the strength of a nucleophile affect the rate of a reaction?
1. Strength of nucleophile: Generally, the strength of the nucleophile does not have any effect on the rate of the reaction since the rate determining step only involves the substrate molecule. However, when two competing nucleophiles are present, their strength and concentration will influence the product distribution.