What is the difference between energy and entropy?
Enthalpy Entropy Gibbs Free Energy Enthalpy is the amount of heat energy transferred (heat absorbed or emitted) in a chemical process under constant pressure.: Entropy measures the amount of heat dispersed or transferred during a chemical process.: Gibbs Energy is also known as energy available to initiate a chemical process and is determined under constant pressure and temperature.
What does entropy have to do with energy?
Entropy is the loss of energy available to do work. Another form of the second law of thermodynamics states that the total entropy of a system either increases or remains constant; it never decreases. Entropy is zero in a reversible process; it increases in an irreversible process.
Is entropy good or bad?
Is entropy good or bad? In general entropy is neither good nor bad. There are many things that only happen when entropy increase, and a whole lot of them, including some of the chemical reactions needed to sustain life, would be considered as good. That likely means that entropy as such is not nearly always a bad thing.
What is the equation for free energy?
The equation is given as: G= H - TS Where, G = Gibbs free energy H = enthalpy T = temperature S = entropy Gibbs free energy is a state function thus it doesn’t depend on the path (i.e., its path independent entity).
How does free energy relate to entropy?
Gibbs free energy is the energy associated with a chemical reaction that can do useful work. It equals the enthalpy minus the product of the temperature and entropy of the system.
Does entropy change free energy?
Spontaneous reactions release free energy as they proceed. Recall that the determining factors for spontaneity of a reaction are the enthalpy and entropy changes that occur for the system. The free energy change of a reaction is a mathematical combination of the enthalpy change and the entropy change.
How does enthalpy and entropy affect free energy?
The free energy change combines the enthalpy change and the entropy change together, along with the temperature, to produce a quantity that can be used to determine if a process is spontaneous or not.
How does entropy affect energy?
The more energy that is lost by a system to its surroundings, the less ordered and more random the system is. Scientists refer to the measure of randomness or disorder within a system as entropy. High entropy means high disorder and low energy (Figure 1).
Why free energy is not possible?
Free energy machines do not work. No machine can create energy out of nothing, as this would violate the law of mass-energy conservation, which is fundamental and universal. The law of mass-energy conservation states that mass-energy can never be created or destroyed.
Which reaction results an increase in entropy?
When a substance goes from a solid to a gas (sublimation) or from a liquid to a gas (evaporation), entropy increases. Likewise, when a solid dissolves in water, entropy increases.
What does Gibbs free energy depend on?
The Gibbs free energy ΔG depends primarily on the reactants' nature and concentrations (expressed in the ΔGo term and the logarithmic term of Equation 1.11, respectively).
What happens to enthalpy when entropy increases?
This means if the energy is added, the enthalpy increases. If the energy is given off, then the enthalpy of the system decreases....Difference Between Enthalpy And Entropy.EnthalpyEntropyIt is the sum total of all the energies inside the system.It increases with the increase in temperature.Symbolized as H.Symbolized as S.4 more rows
Why is enthalpy not free energy?
Free Energy vs Enthalpy Enthalpy gives the total energy of a system that can be converted to heat. Free energy gives the energy that can be converted to mechanical work of the system. Enthalpy gives the energy that can be converted to non-mechanical work of the system.
Does higher entropy mean less energy?
Entropy is a measure of randomness and disorder; high entropy means high disorder and low energy.
Is energy destroyed in entropy?
Entropy, as thermal disorder, is always generated (produced), in all processes without exception, and cannot be destroyed (no “thermal order”) by any means. This should not be confused with local entropy change that could increase or decrease due to entropy transfer.
What does it mean when entropy increases?
Entropy increases when a substance is broken up into multiple parts. The process of dissolution increases entropy because the solute particles become separated from one another when a solution is formed. Entropy increases as temperature increases.
How does free energy and entropy changes during adsorption?
The adsorption process is a spontaneous process, where the Gibbs free energy decreases. During this process, the gas molecular entropy and the enthalpy of the system decrease, which indicates an enthalpy-driven process.
Is Gibbs free energy the 2nd law of thermodynamics?
Its value is usually expressed in Joules or Kilojoules. Gibbs free energy can be defined as the maximum amount of work that can be extracted from a closed system....Second Law of Thermodynamics.∆H<0∆H>0∆S>0Spontaneous at all temperatures (∆G<0)Spontaneous only at high temperature (when T∆S is high)1 more row
What happens to enthalpy when entropy increases?
This means if the energy is added, the enthalpy increases. If the energy is given off, then the enthalpy of the system decreases....Difference Between Enthalpy And Entropy.EnthalpyEntropyIt is the sum total of all the energies inside the system.It increases with the increase in temperature.Symbolized as H.Symbolized as S.4 more rows
What increases and decreases entropy?
In a chemical reaction, when we increase temperature of any substance, molecular motion increase and so does entropy. Conversely, if the temperature of a substance is lowered, molecular motion decrease, and entropy should decreases.
How to interpret entropy increase?
If you increase the energy, there are more ways to spread the energy across the N particles, so it is more difficult to guess the actual microstate. If the average energy U = ∑ i p i E i increases, the microstates with higher energy will be more likely, and since there are a greater number of them, the entropy should increase.
Why does a system tend to minimise energy?
That is why the system tends to minimise energy: because it increases the overall entropy! But there is of course a tradeoff: If the system gives away energy, it will also lose some entropy. Equilibrium occurs when the system's lost entropy is equal to the environment's gained entropy.
Is entropy a measure of energy density?
A paper that my professor once showed me makes it clear that entropy isn't a measure of energy density/distribution... rather, it's more of a statistical observation.
Why does entropy increase with higher temperature?
5. Entropy will increase because the higher temperature will increase the energy of the molecules. As a result, the molecules will have more disorder than those at lower temperatures.
What is the second property of entropy?
The second property is the measure of entropy (S), which is a measure of disorder or randomness in the system. In nature, a messy room is far more favored than a neat, ordered room, and when disorder increases, we have +S.
What Is Free Energy?
Chemical reactions are all around us. They are even inside our body. In fact, the human body is a mass of thousands of chemical reactions. If you are like me, the one you are really familiar with is eating and digesting, scientifically called cellular respiration. I love this chemical reaction. This is where we eat delicious food that our body breaks down to form the products of carbon dioxide and water. This chemical reaction gives out energy that we can use.
What is the sign of a spontaneous reaction?
Now it turns out that for a spontaneous reaction, the sign of Delta G must be negative. Knowing this, what effect does enthalpy and entropy have on spontaneity? Let us first look at enthalpy: an exothermic reaction is more likely to be spontaneous; if Delta H is negative, this makes it more likely that Delta G will also be negative and, therefore, spontaneous. And now, let's look at entropy: if a reaction causes an increase in randomness of the system, it is more likely to be spontaneous; a positive Delta S term will also mean that Delta G is more likely to be negative.
What is the Gibbs equation for change in G?
The Gibbs free energy equation we will be working with is Delta or change in G is equal to change in enthalpy minus temperature multiplied by the change in entropy.
How is Gibbs free energy calculated?
As we can see from our equation, Gibbs free energy is calculated from the changes in enthalpy and entropy, as well as the temperature at which the reaction is carried out at. Let us quickly remind ourselves about these important thermodynamic quantities.
Why is Delta G negative?
Because, remember, a negative Delta G is needed for a reaction to be spontaneous. Where things get a little tricky is when you have both enthalpy and entropy with the same sign. And here it all depends on the effect on entropy by temperature.
Who developed the molecular scale statistical model that related the entropy of a system to the number of?
Following the work of Carnot and Clausius, Ludwig Boltzmann developed a molecular-scale statistical model that related the entropy of a system to the number of microstates possible for the system. A microstate ( W) is a specific configuration of the locations and energies of the atoms or molecules that comprise a system:
Does spontaneity depend on temperature?
As we’ve seen previously, the spontaneity of a process may depend upon the temperature of the system. Phase transitions, for example, will proceed spontaneously in one direction or the other depending on the temperature of the substance. Likewise, chemical reactions can exhibit temperature dependent spontaneities. Considered:
What is the entropy of the universe?
Entropy is a quantitative expression for the randomness or disorder in a system and is represented by the symbol S. Entropy has already been discussed in quite some detail while describing Second Law of Thermodynamics earlier. According to this law, ‘the entropy of the universe tends towards a maximum’.
What is the free energy change of a chemical reaction?
The free energy change (∆G) of a chemical reaction is a function of its displacement from equilibrium. “The farther a reaction is poised away from equilibrium, the more free energy is available as the reaction proceeds towards equilibrium”. When a reaction is at equilibrium, ∆G is zero and no further work can be done.
What is the enthalpy of a reaction?
In simpler words, enthalpy is the total heat content of a system. It reflects the number and kinds of chemical bonds in the reactants and products. Like internal energy, enthalpy is also a function of state and therefore, it is not possible to quantify the absolute enthalpy. However, a change in enthalpy (∆H) accompanying a process can be measured accurately. Thus,
What is the standard free energy change?
The standard free energy change (∆G°’) represents free energy change of a reaction that occurs at pH 7 and 25°C under conditions when both reactants and products are at unit concentration i .e., 1M. The actual free energy change (∆G) and standard free energy change (∆G°’) are two different quantities that will not necessarily match each other.
What is Gibbs free energy?
Gibbs Free Energy (G): Free energy is the component of the total energy of a system that is available to do work at constant temperature and pressure and is represented by the symbol G. It is called as Gibbs free energy in honour of Josiah Willard Gibbs (1839-1903), an American mathematician and physical chemist who developed the theory ...
Is Gibbs free energy thermodynamic?
Since Gibbs free energy is also a thermodynamic quantity, it is not possible to quantify its absolute value. However, a change in Gibbs free energy (∆G) accompanying a process can be measured accurately. The unit of Gibbs free energy is joules/mole (or calories/ mole). Gibbs free energy (G) can be defined by combining the enthalpy (H), entropy (S), ...
When a reaction is at equilibrium, what is the magnitude of free energy changes?
When a reaction is at equilibrium, ∆G is zero and no further work can be done. The magnitude of free energy changes is mostly a function of the particular set of conditions for that reaction. Therefore, free energy changes in chemical reactions are compared under standard reaction conditions.
How to find absolute entropy of a substance at a given temperature?
To find the entropy of a substance at a given temperature, we cool it as close to 0 K as possible. We then heat in small increments, measure qand T, and calculate DSfor each increment. The sum of these DSvalues gives the absoluteentropy at the temperature of interest.
What crystal has zero entropy?
A perfect crystal has zero entropy at absolute zero.
How does a process become spontaneous?
For a process to be spontaneous, a decreasein the entropy of the system must be offset by a larger increase in the entropy of the surroundings.
What is the relationship between the number of microstates in a system and the entropy of the system?
The number of microstate s (W) in a system is related to the entropy(S) of the system. A system with fewermicrostates has lower entropy. A system with moremicrostates has higher entropy. All spontaneous endothermic processes exhibit an increase in entropy.
What is the standard molar entropy of a substance?
S° is the standard molar entropyof a substance, measured for a substance in its standard state in units of J/mol·K. The conventions for defining a standard state include: • 1 bar for gases • 1 mol/Lfor solutions, and • the pure substance in its most stable form for solids and liquids.
What is the microstate of energy?
quantized, a system of particles also has different allowed energy states. • Each quantized energy state for a system of particles is called a microstate.
How does Ne gas work?
The gas does work on its surroundings, absorbing a tiny increment of heat, q, from the heat reservoir. This simulates a reversible process, since it can be reversed by replacing the grain of sand.
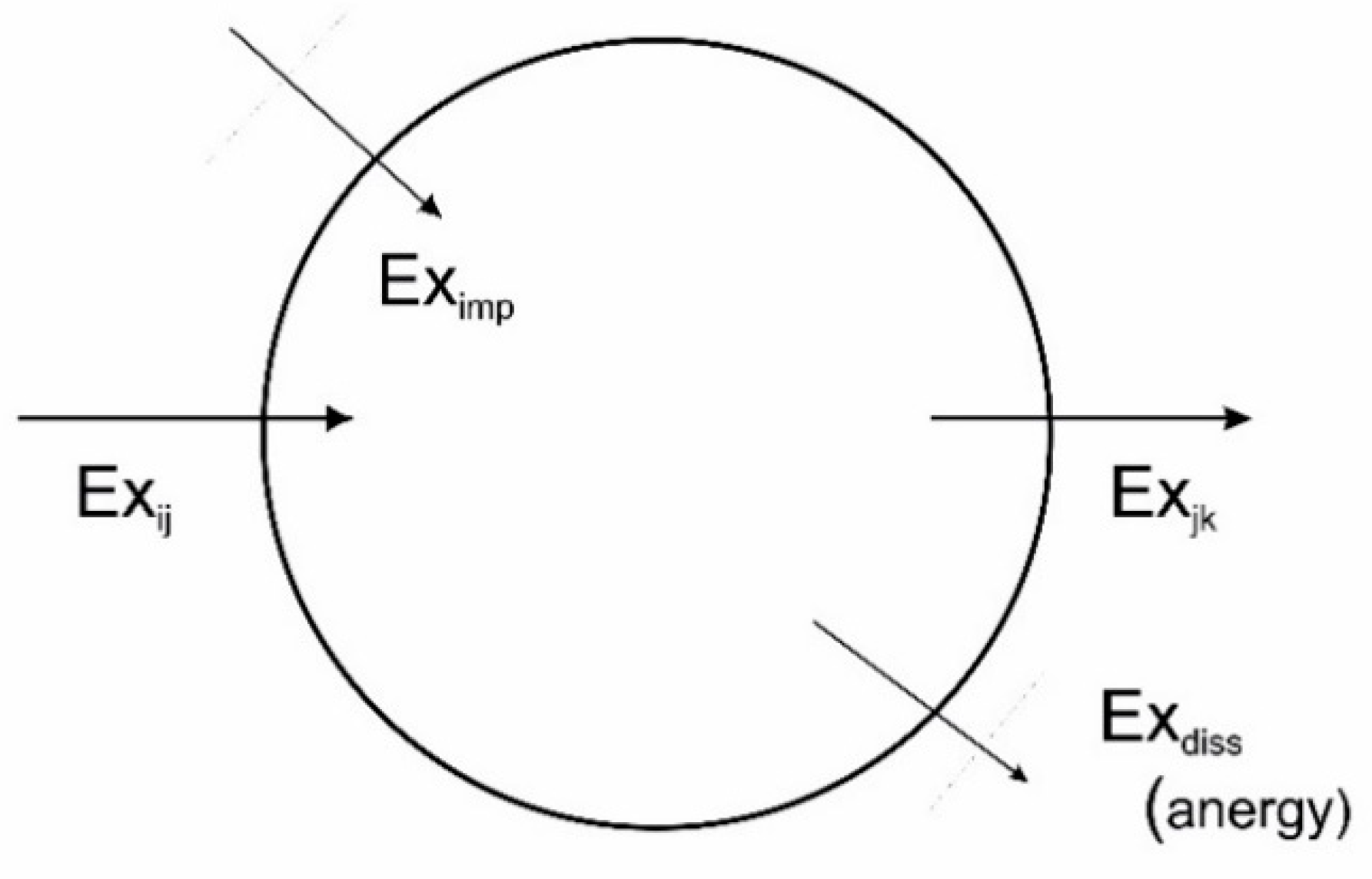
What is the energy released by entropy?
There is no energy "released by entropy". The entropy portion determines how much choice there is of running a process with respect to work vs heat. In some processes, the work you can extract is larger than the enthalpy provided by the process (the additional energy comes from cooling down the surroundings).
What is the formula for Gibbs free energy?
Given that the formula for Gibbs free energy is Δ G = Δ H − T Δ S , it is quite clear that a change in enthalpy is not a change in Gibbs free energy. Rather, the entropy seems to also be able to contribute work.
What is Gibbs energy?
The Gibbs energy is a measure of the maximum non-PV work a system can do (if the Gibbs energy is negative), or the minimum non-PV work that has to be done on the system to make the process go forward (if the Gibbs energy is positive). The Gibbs energy is practical to use for constant pressure processes ...
Is thermodynamics intuitive?
I have not found an intuitive way to understand any of thermodynamics. Its beauty is the high level of abstraction, which might make it one of the less intuitive topics. I get surprised everytime I set it aside and then think about it again. One way to deal with this is to have a couple of examples you are comfortable with and where you know the proper description of what is going on. Then, you can return to these as you are looking at a situation that is new to you (or one you have forgotten about).
Is constant pressure a non-PV process?
That is true for a constant-pressure process in the absence of non-PV work. In the presence of non-PV work, you would have to add that to the heat exchanged (with the proper conventions for the +/- signs of heat and work).
Is Gibbs energy used for constant pressure?
The Gibbs energy is practical to use for constant pressure processes (where we know what the PV-work is for a given process). But, I've also learnt that the heat released when I burnt ethanol was due to the enthalpy of the reaction. That is true for a constant-pressure process in the absence of non-PV work.

Entropy
Standard Entropy
Spontaneous Reactions
Free Energy
Calculating Free Energy
Temperature and Free Energy
Changes of State and Free Energy
- At the temperature at which a change of state occurs, the two states are in equilibrium with one another. For an ice-water system, equilibrium takes place at 0°C, so is equal to 0 at that temperature. The heat of fusion of water is known to be equal to 6.01 kJ/mol, and so the Gibbs free energy equation can be solved for the entropy change that occu...
Equilibrium Constant and