Explain how formation of an enzyme-substrate complex increases the rate of reaction. When an enzyme comes into contact with a substrate that fits its active site, it will bind to it and form an enzyme-substrate complex. This binding alters some of the bonds in the substrate to be slightly weaker or easier to break.
How does formation of an enzyme-substrate complex increase the rate of reaction?
Explain how formation of an enzyme-substrate complex increases the rate of reaction. When an enzyme comes into contact with a substrate that fits its active site, it will bind to it and form an enzyme-substrate complex. This binding alters some of the bonds in the substrate to be slightly weaker or easier to break.
What happens when an enzyme binds with a substrate?
In the above illustration, enzyme (E) binds with substrate (S), forming an enzyme-substrate complex (ES). Following the ES complex formation, E and S interaction takes place, resulting in an enzyme product (EP) complex.
What is an enzyme substrate complex?
Enzyme-substrate Complex In a chemical reaction, the step wherein a substrate binds to the active site of an enzyme is called an enzyme-substrate complex. The activity of an enzyme is influenced by certain aspects such as temperature, pH, co-factors, activators, and inhibitors.
Why does substrate concentration not increase the rate of reaction?
The reason that increasing the substrate concentration of an enzymic reaction does not increase the reaction rate beyond a certain maximum (Vmax) is that the reaction rate is dependent on the concentration of enzyme-substrate complex, not its rate of formation.
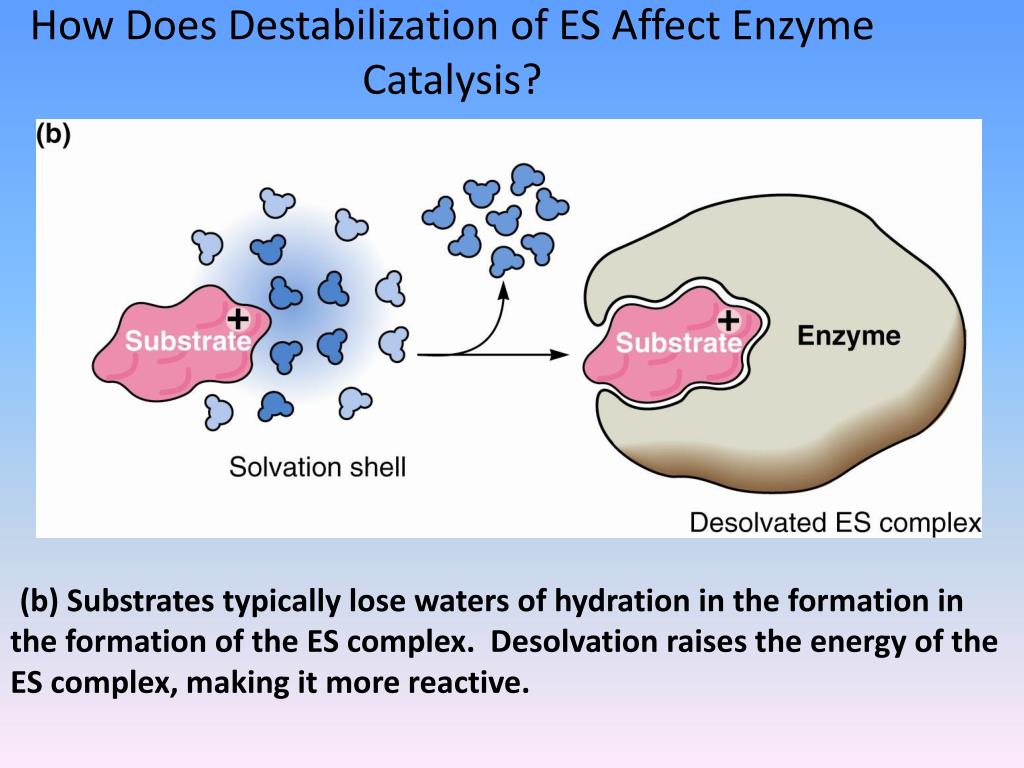
How does the formation of the enzymes substrate complex?
A substrate enters the active site of the enzyme. This forms the enzyme-substrate complex. The reaction then occurs, converting the substrate into products and forming an enzyme products complex. The products then leave the active site of the enzyme.
How does the formation of an enzyme-substrate complex lower the activation energy of a reaction?
It does this by forming an enzyme-substrate (ES) complex. 1. The enzyme may hold the substrates in such a way as to distort the substrate bonds closer to their form in the transition state. This reduces the amount of energy needed to complete the transition.
How do enzymes increase the rate of reaction a level?
Increasing [enzyme] initially increases rate. Similar to increasing the concentration of substrate in a reaction, increasing the number of enzymes increases the rate by increasing the amount of collisions between enzymes and substrates.
Do enzymes increase the rate at which substrate is converted to product?
The enzyme reduces the energy barrier required to activate the substrate, allowing more substrates to become activated, which increases the rate of product formation. Note that the energy difference between the substrate and the product is not changed by the enzyme.
How does substrate concentration affect the rate of an enzyme controlled reaction?
Substrate concentration: Increasing substrate concentration also increases the rate of reaction to a certain point. Once all of the enzymes have bound, any substrate increase will have no effect on the rate of reaction, as the available enzymes will be saturated and working at their maximum rate.
What is an enzyme-substrate complex?
Definition. A non-covalent complex composed of a substrate bound to the active site of the enzyme. Supplement. The enzyme-substrate complex is formed during a chemical reaction.
Why does rate of reaction increase with substrate concentration?
Increasing Substrate Concentration increases the rate of reaction. This is because more substrate molecules will be colliding with enzyme molecules, so more product will be formed.
How do enzymes affect the rate of reaction?
Enzymes are biological catalysts. Catalysts lower the activation energy for reactions. The lower the activation energy for a reaction, the faster the rate. Thus enzymes speed up reactions by lowering activation energy.
What is the relationship between the reaction rate and the substrate concentration?
For an enzyme-catalysed reaction, there is usually a hyperbolic relationship between the rate of reaction and the concentration of substrate, as shown below: (A) At low concentration of substrate, there is a steep increase in the rate of reaction with increasing substrate concentration.
How can one increase or decrease the rate of enzyme reactions?
Answers. If the concentration of the substrate is low, increasing its concentration will increase the rate of the reaction. An increase in the amount of enzyme will increase the rate of the reaction (provided sufficient substrate is present).
How does an enzyme react with a substrate?
An enzyme attracts substrates to its active site, catalyzes the chemical reaction by which products are formed, and then allows the products to dissociate (separate from the enzyme surface). The combination formed by an enzyme and its substrates is called the enzyme–substrate complex.
What happens when a substrate binds to an enzyme?
When an enzyme binds its substrate, it forms an enzyme-substrate complex. This complex lowers the activation energy of the reaction and promotes its rapid progression by providing certain ions or chemical groups that actually form covalent bonds with molecules as a necessary step of the reaction process.
How does an enzyme increase the rate of a reaction quizlet?
Enzymes speed up reactions by lowering activation energy, the lower the activation energy for a reaction, the faster the rate.
What two factors affect the rate of enzyme action?
Several factors affect the rate at which enzymatic reactions proceed - temperature, pH, enzyme concentration, substrate concentration, and the presence of any inhibitors or activators.
What is rate of reaction a level?
The measure of a change in concentration of the reactants and products per time unit. Rate = Moles of product formed time elapsed. Rate = Moles of reactants transformed time elapsed. Reactive Collision.
What is it called when a substrate binds to a specific enzyme?
When a substrate binds to a specific enzyme, it is called an enzyme-substrate complex.
What is the step wherein a substrate binds to the active site of an enzyme?
In a chemical reaction, the step wherein a substrate binds to the active site of an enzyme is called an enzyme-substrate complex . The activity of an enzyme is influenced by certain aspects such as temperature, pH, co-factors, activators, and inhibitors.
What are the compounds that carry molecules from one enzyme to another called?
These additional, non-proteinaceous substances are referred to as cofactors. The compounds that carry molecules from one enzyme to other are called coenzymes.
What are the properties of enzymes?
Enzyme Properties. All types of biological units require specific enzymes for specific reactions. The role of enzymes is to accelerate or catalyze the reaction, while remaining unchanged throughout the process. This action is achieved by reducing the activation energy required to initiate the chemical reaction.
How does the rate of reaction vary?
The rate of reaction varies significantly when performed with or without enzymes. Each enzyme has a specific substrate, which is determined by its active site. As mentioned already, these compounds are proteins that have a globular structure. The amino acid arrangement in the active site is such that it is specific for recognizing only one type ...
What are the three basic components of a chemical reaction?
Thus, for any type of chemical reaction, there are three basic components, viz., substrate, enzyme, and product.
Do enzyme inhibitors block substrate binding?
Studies clearly indicate that inhibitor molecules attach to the same active site, thus, blocking the binding of substrates. Enzyme inhibitors are medically employed as drugs and medicines for killing disease causing pathogens.
Why is the enzyme substrate complex important?
The enzyme substrate complex is extremely important for a number of reasons. First, the enzyme substrate complex is only temporary. This means that once the substrate has changed, it can no longer bind to the enzyme. The products are released and the enzyme is ready for another substrate molecule. A single enzyme can operate repeatedly millions ...
What happens to the enzyme substrate complex when mutations are beneficial?
In mutations that are beneficial to the organism, the enzyme substrate complex is changed in a way that effects the output of product or the function of the enzyme as a whole. This change in the organism is only beneficial if it somehow helps the organism reproduce more.
How do enzymes produce products?
Some enzymes produce a single product from two substrate molecules. In these enzymes, the substrates are loaded into the active site, the enzyme substrate complex is formed, and a single product is released.
How does amylase work?
Amylase uses one substrate molecule of amylose and a cofactor of one water molecule to produce an enzyme substrate complex. The complex severely reduces the amount of energy required to start the reaction, which increases the time in which it happens.
How do enzymes work?
Enzymes are complex molecules, like little machines meant for one purpose. Built out of a chain of amino acids, this long string experiences interactions between the different amino acids and twists and turns into complex structures. These structures can operate like hinges, wedges, and all sorts of other shapes intended to speed certain reactions. Different mutations give rise to slightly different forms of enzyme. In mutations that are beneficial to the organism, the enzyme substrate complex is changed in a way that effects the output of product or the function of the enzyme as a whole. This change in the organism is only beneficial if it somehow helps the organism reproduce more.
What is an enzyme substrate complex?
The enzyme substrate complex is a temporary molecule formed when an enzyme comes into perfect contact with its substrate. Without its substrate an enzyme is a slightly different shape. The substrate causes a conformational change, or shape change, when the substrate enters the active site. The active site is the area of the enzyme capable ...
What happens when too much ATP is produced?
In this way, when too much ATP is produced, the enzyme shuts off. This is known as feedback inhibition, or the ability to self-regulate. In the same way, the enzymes can be reactivated by the presence of adenosine diphosphate ADP, an ATP that has used a phosphate group to provide energy to a process or reaction.
What is the concentration of substrate needed to reach saturation?
The concentration of substrate needed to reach this saturation (more easily measurable as that to achieve half maximum initial velocity ) is another characteristic of the enzyme (and substrate) and can be thought of as reflecting the affinity of the enzyme for substrate (the dissociation constant). The hyperbolic nature of the rate curve is similar to that for binding of small molecules to non-catalytic proteins such as the oxygen-carrier, myoglobin, and hormone receptor proteins.
Why does increasing the substrate concentration of an enzyme reaction not increase the reaction rate beyond a certain maximum?
The reason that increasing the substrate concentration of an enzymic reaction does not increase the reaction rate beyond a certain maximum (Vmax) is that the reaction rate is dependent on the concentration of enzyme-substrate complex, not its rate of formation.
What does substrate concentration affect?
What the substrate concentration does affect is the position of equilibrium for the formation of ES from E and S , i.e. how much enzyme is converted into complex. However there is obviously a maximum, approached asymptotically, equivalent to the total enzyme present. This explains† the hyperbolic nature of the curve for reaction rate.
What is the steady state assumption?
A key assumption — the steady state assumption — in Michaelis and Menten’s original derivation is that there is that there is a considerable excess of substrate over enzyme so that the substrate is in instantaneous equilibrium with the ES complex. So, in effect, at a particular concentration of substrate the concentration of ES is constant. The implication in the question that the rate of formation of this complex would determine the reaction rate is incorrect.
What is the meaning of "back up"?
Making statements based on opinion; back them up with references or personal experience.
What is the Michaelis-Menten treatment?
The Michaelis–Menten treatment considers the initial velocity of reactions, at which time little product has formed so that it is assumed that the back reaction from product can be ignored, simplifying the situation to a consideration of the rates of three reactions:
Do all enzymes have hyperbolic kinetics?
Just as not all proteins in this latter class (e.g. haemoglobin) show simple hyperbolic binding of their ligand, so not all enzymes (e.g. allosteric enzymes) show hyperbolic kinetics. But that’s another story, which has been told many times on this site.
How does an enzyme catalyze a substrate?
The enzyme can perform its catalysis mechanism on the substrate only when it is in the enzyme-substrate complex. It is in this structure that the enzyme is able to increase the rate of the reaction either by causing bond distortion or polarisation to lower the activation energy, creating an alternative pH environment for the substrate within the active site which are the optimum conditions for that reaction, locating the substrates close to each other to make contact (and so the reaction) more likely or by creating an alternative state with the substrate as this alternative transition state has a lower activation to be overcome in order to allow the reaction to continue.
What is the enzyme involved in a rate limiting step of a reaction?
The enzyme is normally involved in a rate limiting step of a reaction as the Enzyme-Substrate complex provides an alternative transition state for the reaction path to follow. This means that a reaction will occur at its maximum rate when there's a maximum number of ES complexes formed (i.e. no empty active sites ).
How do enzyme inhibitors affect the ES complex?
They can affect the ES complex in a number of ways, such as: by preventing the formation of the ES complex ( competitive and irreversible inhibitors) or they prevent the catalytic activity of the enzyme once in the ES complex ( non competitive and uncompetitive inhibitors). This shows how important the formation of enzyme-substrate complexes are for many biological processes as enzyme inhibitors are a major area for drug development. Drugs such as Penicillin, Aspirin, Tamiflu and many more are examples of the medications used to treat to disease which act as inhibitors on certain enzymes.
What are some examples of enzyme inhibitors?
Drugs such as Penicillin, Aspirin, Tamiflu and many more are examples of the medications used to treat to disease which act as inhibitors on certain enzymes.
What is the active site of an enzyme?
Every enzyme is equipt with a 'cleft' in its structure, which is complementary to a particular substrate. This is known as the active site. As well as shape, the specificity of the active site is developed further via the amino acid R-Groups within the site.
What is the ES complex?
The ES complex is the state in, which the active site of the enzyme is non-covalently bound to the substrate molecule (s). This is where the chemical reaction occurs: ENZYME + SUBSTRATE -> ENZYME SUBSTRATE COMPLEX -> ENZYME + PRODUCT.