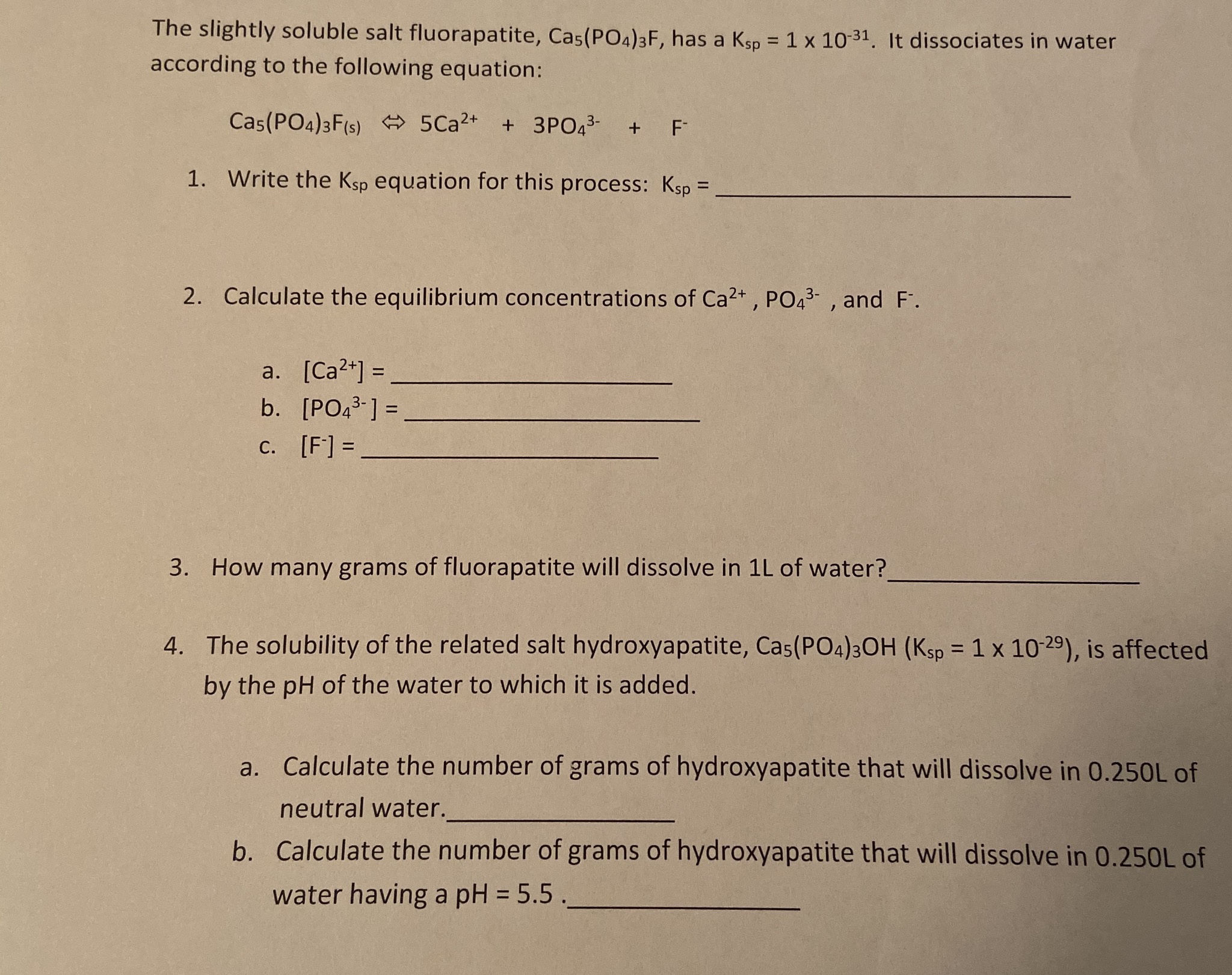
The solubility product constant (Ksp) describes the equilibrium between a solid and its constituent ions in a solution. The value of the constant identifies the degree to which the compound can dissociate in water. The higher the Ksp, the more soluble the compound is.
How do you find solubility from KSP?
How do you find solubility from KSP? Set up an ICE problem (Initial, Change, Equilibrium) in order to use the Ksp value to calculate the concentration of each of the ions. The concentration of the ions leads to the molar solubility of the compound.
How to calculate Ksp value?
Solution:
- Write the chemical equation: Cu (OH) 2 ⇌ Cu 2+ + 2 OH¯
- Write the K sp expression: K sp = [Cu 2+] [OH¯] 2 1.6 x 10¯ 19 = (s) (2s) 2 s = 3.42 x 10¯ 7 M
- This is an important point: what we have calculated is 's' and it is NOT the [OH¯]. ...
- The natural contribution of hydroxide from water molecules ionizing is 1.00 x 10¯ 7 M. ...
What factors affect KSP?
What factors affect the value of Ksp? Some important factors that have an impact on the solubility product constant are: The common-ion effect (the presence of a common ion lowers the value of Ksp). The diverse-ion effect (if the ions of the solutes are uncommon, the value of Ksp will be high).
How do you calculate KSP from molar solubility?
- Set up an ICE problem (Initial, Change, Equilibrium) in order to use the Ksp value to calculate the concentration of each of the ions.
- The concentration of the ions leads to the molar solubility of the compound.
- Use the molar mass to convert from molar solubility to solubility.
Why does higher Ksp mean more soluble?
The solubility product is a kind of equilibrium constant and its value depends on temperature. Ksp usually increases with an increase in temperature due to increased solubility. Solubility is defined as a property of a substance called solute to get dissolved in a solvent in order to form a solution.
What does a low Ksp mean?
Explanation: K sp is often written in scientific notation like 2.5 x 10−3. The larger the negative exponent the less soluble the compound is in solution. The larger the real value of the Ksp the more soluble the compound is in solution 2.5 x 10−3 > 2.5 x 10−6.
Does higher Ksp mean more concentration?
The higher the value for Ksp, the higher the concentration of these ions at equilibrium, which means that more of the solid must have dissolved.
Does lower Ksp mean it will precipitate first?
If the solution contained about equal concentrations of Cl– and I–, then the silver salt with the smallest Ksp (AgI) would precipitate first.
What does the Ksp value tell you?
The solubility product constant, Ksp, is the equilibrium constant for a solid substance dissolving in an aqueous solution. It represents the level at which a solute dissolves in solution. The more soluble a substance is, the higher the Ksp value it has.
What is Ksp and why is it important?
What is K s p in chemistry? The solubility product constant, or K s p , is an important aspect of chemistry when studying solubility of different solutes. K s p represents how much of the solute will dissolve in solution, and the more soluble a substance is, the higher the chemistry K s p value.
Does a higher Ksp mean more precipitate?
Explanation: 1) If Solubility product is larger than the ionic product then no precipitate will form on adding more solute because unsaturated solution is formed. 2) If Solubility product is smaller than the ionic product then excess solute will precipitate out because of the formation of super saturated solution.
What does it mean when Ksp is greater than 1?
A Ksp value greater than 1 means that the dissociation of the solid compound into its constituent ions is highly favorable.
How does KSP relate to precipitation?
If Q = Ksp, a precipitate will form. If Q > Ksp, a precipitate will form. Note that precipitation may not happen immediately if Q is equal to or greater than Ksp. A solution could be supersaturated for some time until precipitation occurs.
How do you tell what will precipitate first from KSP?
0:004:50Example: Determining Which Solid Will Precipitate First ... - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd then we could just go by the the KSP value and see that well this KSP value is smaller. MeanMoreAnd then we could just go by the the KSP value and see that well this KSP value is smaller. Mean that we're gonna reach this first. And so lead carbonates will precipitate first.
What does low water solubility mean?
Water solubility is measured in mg/L, the weight of pesticide (in milligrams) that will dissolve in one liter of water (L). Low water solubility: less than 10 mg/L or 10 ppm1. Moderate water solubility: 10-1,000 mg/L or 10-1,000 ppm1.
What is the lowest Ksp value?
▶"Cobalt(III) sulfide" has the lowest Ksp value.
Which has lowest Ksp?
The hydroxide of alkaline earth metal, which has the lowest value of solubility product (K(sp)) at normal temperature (25^(@)C) is : UPLOAD PHOTO AND GET THE ANSWER NOW! Solution : `Be(OH)_(2)` has lowest solubility and hence lowest solubility product.
What would decrease Ksp?
1)Ksp will decrease by addition of Pb in the form of Pb(NO3)2 P b ( N O 3 ) 2 or by additin of Cl− in the form of KCl.
What is the relationship between Ksp and solubility?
Ksp defines how soluble a solute it. If Ksp is large, that molecule dissolves readily into solution. It Ksp is small, it barely dissolves.
How to find molar solubility from Ksp?
To find molar solubility from Ksp, start with the Ksp equation. The concentrations are substituted with X based on stoichiometry, then X is solved...
How does the value of Ksp affect molar solubility?
If Ksp is large, the molar solubility of a solute is generally going to be large. If Ksp is small however, the solute will also have a small molar...
How to write a solubility product constant expression?
A solubility product constant expression comes from the equilibrium expression. For a reaction AyBz to y A + z B, the expression is Ksp=A^y × B^z
What is the relationship between solubility and K s p?
Considering the relation between solubility and K s p is important when describing the solubility of slightly ionic compounds. However, this article discusses ionic compounds that are difficult to dissolve; they are considered "slightly soluble" or "almost insoluble." Solubility product constants ( K s q) are given to those solutes, and these constants can be used to find the molar solubility of the compounds that make the solute. This relationship also facilitates finding the K s q of a slightly soluble solute from its solubility.
What is the KSP of copper bromide?
The Ksp of copper (I) bromide, CuBr, is 6.3 × 10 –9. Calculate the molar solubility of copper bromide.
What is the solubility of a solution?
Solubility is defined as the maximum amount of solute that can be dissolved in a solvent at equilibrium. Equilibrium is the state at which the concentrations of products and reactant are constant after the reaction has taken place. The solubility product constant ( K s p) describes the equilibrium between a solid and its constituent ions in a solution. The value of the constant identifies the degree to which the compound can dissociate in water. The higher the K s p, the more soluble the compound is. K s q is defined in terms of activity rather than concentration because it is a measure of a concentration that depends on certain conditions such as temperature, pressure, and composition. It is influenced by surroundings. K s p is used to describe the saturated solution of ionic compounds. (A saturated solution is in a state of equilibrium between the dissolved, dissociated, undissolved solid, and the ionic compound.)
What is saturated solution?
A saturated solution is a solution at equilibrium with the solid. Thus:
When we know the K s p value of a solute, we can figure out if a?
When we know the K s p value of a solute, we can figure out if a precipitate will occur if a solution of its ions is mixed. Below are the two rules that determine the formation of a precipitate.
What Factors Affect K s p?
In this section, we discuss the main factors that affect the value of the solubility constant.
What Is K s p?
K s p is known as the solubility constant or solubility product. It’s the equilibrium constant used for equations when a solid substance is dissolving in a liquid/aqueous solution. As a reminder, a solute (what is being dissolved) is considered soluble if more than 1 gram of it can be completely dissolved in 100 ml of water.
How Do You Calculate K s p?
For most chemistry classes, you’ll rarely need to solve for the value of K s p; most of the time you’ll be writing out the expressions or using K s p values to solve for solubility (which we explain how to do in the “Why Is K s p Important” section).
What is the common ion effect?
The common ion effect states that when two solutions that share a common ion are mixed, the solute with the smaller K s p value will precipitate first. For example, say BiOCl and CuCl are added to a solution.
How many solubility rules are there?
Inconsolable that you finished learning about the solubility constant? Drown your sorrows in our complete guide to the 11 solubility rules.
What is the difference between k and c?
k is Henry’s law constant. c is the concentration of gas in the liquid. Henry’s law shows that, as partial pressure decreases, the concentration of gas in the liquid also decreases, which in turn decreases solubility. So less pressure results in less solubility, and more pressure results in more solubility. You can see Henry’s law in action ...
What is the equilibrium constant for a solid substance that dissolves in an aqueous solution?
The solubility product constant, K s p , is the equilibrium constant for a solid substance dissolving in an aqueous solution. It represents the level at which a solute dissolves in solution. The more soluble a substance is, the higher the K s p value it has.
Why are solids not included in equilibrium constant expressions?
Solids are not included when calculating equilibrium constant expressions, because their concentrations do not change the expression; any change in their concentrations are insignificant, and therefore omitted. Hence, Ksp represents the maximum extent that a solid that can dissolved in solution.
What is the salt effect?
Salt Effect (diverse ion effect): Having an opposing effect on the K s p value compared to the common ion effect, uncommon ions increase the K s p value. Uncommon ions are ions other than those involved in equilibrium.
Do highly soluble ionic compounds have ionic activities?
For highly soluble ionic compounds the ionic activities must be found instead of the concentrations that are found in slightly soluble solutions.
What does KSP mean in equilibrium?
Similar to other equilibrium expressions you may have encountered, the symbol K is used for the equilibrium constant. We use Ksp to show that this equilibrium is specific to solubility. The proper term for Ksp is solubility product constant, or solubility constant. It has no units!
How to calculate KSP?
Ksp can be calculated by writing the law of mass action for a solution and inputting the concentration of ions into the equation. This can be done using ion concentrations or based on solubility data. The concentrations of ions can be determined given only the Ksp value for a solution at equilibrium.
How does temperature affect solubility?
Generally, increasing temperature increases a substance's solubility. When you run cold water through a faucet or decrease the flow to a trickle, the dissolved chemicals become less soluble and form solids - a.k.a. pesky buildup.
When is a substance considered insoluble?
A substance is considered to be insoluble when its solubility is less than 0.1 g per 100 g solvent. When a solution of a given volume has a maximum amount of a solid dissolved in it, it is said to be saturated. For solutions at equilibrium, the law of mass action is written only in terms of the ions produced.
Why do we leave out the solid form of AB2?
Remember that when writing laws of mass action you don't have to consider solids or liquids because their quantities cannot be expressed in terms of concentration. So, we get to leave out the solid form of AB2.
Is AgCl in equilibrium?
We know that AgCl is in a dynamic equilibrium with its constituent ions, Ag^+ and Cl^-, which can be expressed as AgCl (s) <==> Ag^+ (aq) + Cl^- (aq).
When a solution of a given volume has the maximum amount of a solid dissolved in it, it is?
When a solution of a given volume has the maximum amount of a solid dissolved in it, it is said to be saturated. For soluble compounds, saturation occurs at high concentrations. For insoluble compounds, saturation happens at very low concentrations.
How does solubility affect temperature?
Solubility is affected by multiple factors. Solids becomes more soluble as temperature increases. As pressure increases, gases become more soluble. The common-ion effect describes a decrease in solubility of an ionic compound when the solution already contains an ion that is the same as one from the compound.
What is the difference between molar solubility and unsaturated?
The molar solubility is the number of moles that can be dissolved per liter of a solution until the solution becomes saturated. A saturated solution is a solution in which the maximum amount of solute has been dissolved. An unsaturated solution is a solution in which all solute has dissolved.
What is a saturated solution?
A saturated solutionis a solution in which the maximum amount of solute has been dissolved at a given temperature.
When a slightly soluble ionic compound is placed in water, what is the equilibrium?
When a slightly soluble ionic compound is placed in water, there is an equilibrium between the solid state and the aqueous ions. This is found by the equilibrium constant for the reaction.
Which solvent will best dissolve a polar compound?
Solvent properties: A polar solvent will best dissolve a polar compound while a nonpolar solvent will best dissolve a nonpolar compound.
Where is the equilibrium constant written?
The equilibrium constant can be written where the power on the components is the coefficient in the balanced equation:
What is the solubility product of KSP?
Introduction. The solubility products Ksp 's are equilibrium constants in hetergeneous equilibria (i.e., between two different phases). If several salts are present in a system, they all ionize in the solution. If the salts contain a common cation or anion, these salts contribute to the concentration of the common ion.
What effect decreases the solubility of a sparingly soluble salt?
The common ion effect usually decreases the solubility of a sparingly soluble salt.
Why does NaCl shift out of equilibrium?
Notice: Q sp > K sp The addition of NaCl has caused the reaction to shift out of equilibrium because there are more dissociated ions. Typically, solving for the molarities requires the assumption that the solubility of PbCl 2 is equivalent to the concentration of Pb 2+ produced because they are in a 1:1 ratio.
How does solubility product expression relate to the solubility of an ionic compound?
Consideration of charge balance or mass balance or both leads to the same conclusion. The solubility product expression tells us that the equilibrium concentrations of the cation and the anion are inversely related. That is, as the concentration of the anion increases, the maximum concentration of the cation needed for precipitation to occur decreases—and vice versa—so that Ksp is constant. Consequently, the solubility of an ionic compound depends on the concentrations of other salts that contain the same ions. Adding a common cation or anion shifts a solubility equilibrium in the direction predicted by Le Chatelier’s principle. As a result, the solubility of any sparingly soluble salt is almost always decreased by the presence of a soluble salt that contains a common ion. The exceptions generally involve the formation of complex ions, which is discussed later.
What is the concentration of lead ions in a solution?
The concentration of lead (II) ions in the solution is 1.62 x 10 -2 M. Consider what happens if sodium chloride is added to this saturated solution. Sodium chloride shares an ion with lead (II) chloride. The chloride ion is common to both of them; this is the origin of the term "common ion effect".
What determines the solubility of an ionic compound?
Consequently, the solubility of an ionic compound depends on the concentrations of other salts that contain the same ions. Adding a common cation or anion shifts a solubility equilibrium in the direction predicted by Le Chatelier’s principle.
What is the effect of the common ion effect on the ionization of a weak base?
The common ion effect suppresses the ionization of a weak base by adding more of an ion that is a product of this equilibrium.