Why does PaO2 decrease with altitude?
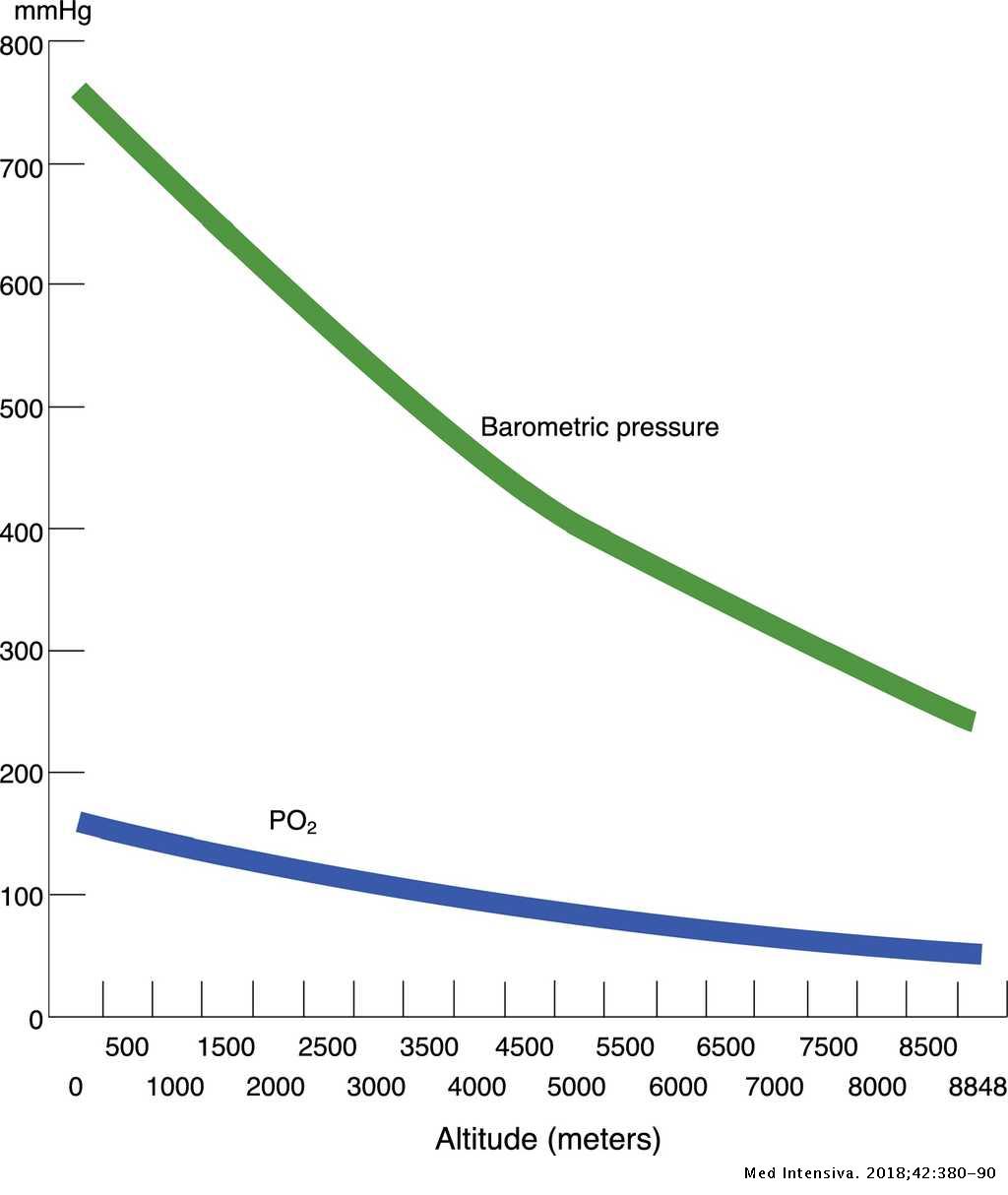
Given that increasing altitude decreases the atmospheric pressure, for any given FiO2 you would expect a lower pAO2 and, consequently, a lower paO2. For example, whereas breathing 100% oxygen at sea level would result in an alveolar pO2 of 663 mmHg, breathing 100% oxygen on Mt.
How does the oxygen level change with altitude?
Oxygen doesn't change, but the partial pressure of oxygen changes with altitude. The weight of the air above us causes the atmospheric pressure, which is roughly 760 mmHg at sea level.
How does atmospheric pressure affect the partial pressure of oxygen?
The partial pressure of oxygen will vary with atmospheric pressure but the partial pressure of water vapor and carbon dioxide are relatively fixed values. Atmospheric pressure of course decreases with altitude. For the same reason, the partial pressure of oxygen in air and the alveoli also decreases as altitude increases.
What is the relationship between oxygen saturation and PaCO2 at altitude?
But the oxygen saturation values at the higher altitudes seem excessively low and this is because one of the adaptations to altitude is an increase in ventilation. This causes PaCO2 to decrease and pH to increase. The decrease in PaCO2 increases PAO2.
What is compensation for DO2?
What is the most important immediate adaptation?
Why is PVR elevated?

How does altitude affect PAO2?
Increasing altitude decreases the atmospheric pressure; thus, for any given FiO2, there is a lower PO2 in the atmosphere and a lower PAO2 in alveoli.
How does the partial pressure of oxygen change with altitude?
As we move upwards, the air becomes less compressed and is therefore thinner. The composition of oxygen in air is around 21%. As the altitude increases, the composition of oxygen doesn't change, but the partial pressure of oxygen starts to decrease.
How does oxygen level change with altitude?
The low amount of oxygen in the air at high altitudes causes high-altitude illness. The amount of oxygen in the air goes down as you climb higher above sea level and becomes very low at altitudes above 8,000 feet. If you travel to a high altitude, you may feel ill because the air has less oxygen in it.
How does a decrease in atmospheric pressure affect oxygen levels PO2 at high altitude?
Oxygen availability and altitude Although the percentage of oxygen in inspired air is constant at different altitudes, the fall in atmospheric pressure at higher altitude decreases the partial pressure of inspired oxygen and hence the driving pressure for gas exchange in the lungs.
Why is oxygen lower at higher altitudes?
This is due to the low air pressure. Air expands as it rises, and the fewer gas molecules—including nitrogen, oxygen, and carbon dioxide—have fewer chances to bump into each other. The human body reacts to high altitudes. Decreased air pressure means that less oxygen is available for breathing.
Does a partial pressure of gases change with altitude?
1 Answer. The partial pressure of all gases will decrease at higher altitudes because the overall pressure decreases.
How much does oxygen saturation decrease with altitude?
Up in Summit, oxygen saturation is around 92%. Visitors coming to Summit from sea level might see their oxygen saturation drop to around 88% or lower before reaching levels typical at this elevation.
What is the oxygen levels at different altitudes?
The percent of oxygen is actually the same at all altitudes, 21%; however, it is 21% of a smaller number as one goes higher. The barometric pressure at sea level is 760 mmHg, and at 10,000 ft, it is 534 mmHg.
Does PO2 decrease in altitude?
Thus, the PO2 of the inspired air decreases with increasing altitude (decreasing barometric pressure) and the body begins to adapt.
What is the partial pressure of oxygen in the lungs at this altitude?
At sea level, percentage of oxygen in the lungs is only 14.5% (in atmosphere it is about 21%). Thus the partial pressure of O2 in the lungs is 100 mmHg. As altitude increases the partial pressure of oxygen in the atmosphere falls but the proportion of oxygen in the atmosphere remains the same.
Where is the partial pressure of oxygen the highest?
the lungsThe partial pressure of oxygen is always highest soon after oxygenation, thus blood returning from the lungs would have a high partial pressure. The superior and inferior vena cavae return deoxygenated blood from the body to the heart, and would have very low oxygen partial pressures.
Does the percentage of oxygen in the air change with altitude?
The percentage of oxygen is the same at sea level as it is at high altitudes, which is roughly 21 percent. However, because air molecules at high altitudes are more dispersed, each breath delivers less oxygen to the body.
Altitude to Oxygen Chart - Hypoxico
At real altitude, the barometric pressure of the atmosphere is significantly less than that of sea-level environments. The result is that oxygen molecules in the air are further apart, reducing the oxygen content of each breath incrementally as one goes up in altitude.
Alveolar O2 and Altitude | PFTBlog
Recently I was trying to explain the effect of altitude on blood oxygenation to somebody with IPF. They had observed that their oxygen saturation was fairly normal at sea level but that they needed to use their supplemental O2 when they went up to 2000 feet and didn’t understand why such a low altitude made that much of a difference.
High-altitude effects on respiratory gases, acid-base balance and ...
Arterial and venous blood were analysed at rest and post exercise for pH, PCO2, and PO2, and bicarbonate ([HCO3-]), base excess (BE), and strong ion difference (SID) were calculated in response to a 10 day sojourn to 3800 m. Pulmonary artery pressures (PAP) were measured at rest. Post exercise sampl …
How does altitude affect the body? - Murdoch University
Dr Brendan Scott. Brendan is a lecturer in strength and conditioning in the School of Psychology and Exercise Science. His research interests include novel resistance training methods, such as using hypoxia (simulated altitude) to improve muscular development.
The correct measurement of oxygen saturation at high altitude
Background: Compared to measurements at sea level, measurement of oxygen saturation by pulse oximetry (SpO 2) at altitude differs fundamentally because of the cyclical course of SpO 2, caused by periodic breathing.Therefore, the determination of a representative SpO 2 value is difficult. In the literature, recommendations for a standardized measurement procedure are missing; different studies ...
Why is PVR elevated?
PVR becomes increased chronically due to hypoxic vasoconstriction. The CO returns to normal with a few days of acclimatization. With continued presence at altitude, vital capacity and FRC is unchanged from baseline, but MV remains elevated.
What is compensation for DO2?
In the long term, compensation includes increases in hemoglobin concentration via hypoxia-mediated renal secretion of erythropoietin, thus maintaining DO2 at the expense of increasing viscosity of blood.
What is the alveolar gas equation?
The alveolar gas equation estimates alveolar oxygen content given a few readily measurable variables. The pAO2 derived from performing the calculation can then be used to discern the degree of shunt present in a patient. Practical simplification of the complex formula allows for the following equation:
Does barometric pressure increase oxygen?
Conversely, increasing the barometric pressure can have significant effects by increasing the amount of dissolved oxygen. It’s for this reason that hyperbaric oxygen therapy has been implemented for the treatment of non-healing wounds, decompression sickness, and carbon monoxide poisoning, among others.
What is the alveolar air equation?
The alveolar air equation only addresses the oxygen concentration in the alveoli. To be of any use oxygen needs to get into the bloodstream. Although diffusing capacity is a measurement of gas exchange efficiency it is not possible to take the DLCO and PAO2, and then predict arterial PO2 (PaO2) with any accuracy.
Why is the accuracy of the pulse oximeter only +/- 3%?
This is because pulse oximeter accuracy is only +/- 3% and blood pH also affects the oxygen dissociation curve. PaO2 must therefore be measured from an arterial blood sample. When this is done you can calculate the difference between alveolar and arterial PO2 (known alternately as the A-a gradient or PAaO2).
What is the normal oxygen level for an airplane?
79.6%. Airplanes that travel at 30,000 to 40,000 feet are usually pressurized to an equivalent altitude of 6000 to 8000 feet and the lower limit of normal for the SaO2 of airplane travelers is usually considered to be between 89% and 91%. Everybody’s arterial oxygen decreases as altitude increases.
Why does oxygen decrease with altitude?
The decrease in the availability of oxygen with increasing altitude is due to the decrease in atmospheric pressure but is also influenced by the relatively constant amounts of water vapor and carbon dioxide in the lung.
When normal values are plugged into the alveolar air equation it looks like this: "An important point is
When normal values are plugged into the alveolar air equation it looks like this: An important point is that when air is inhaled into the lung oxygen is diluted by the water vapor and carbon dioxide that are already there. The partial pressure of oxygen will vary with atmospheric pressure but the partial pressure of water vapor ...
Is 2000 feet lower than sea level?
This means that although the atmospheric pressure at 2000 feet is 7% less than at sea level, alveolar PO2 is 11% less. However, not every individual’s arterial PCO2 and respiratory exchange ratio are “normal” and differences can either increase or decrease the alveolar PO2.
Does atmospheric pressure decrease with altitude?
Atmospheric pressure of course decreases with altitude. For the same reason, the partial pressure of oxygen in air and the alveoli also decreases as altitude increases. But because water vapor and carbon dioxide are relatively constant, the partial pressure of alveolar oxygen decreases faster than the partial pressure of O2 in air.
What is compensation for DO2?
In the long term, compensation includes increases in hemoglobin concentration via hypoxia-mediated renal secretion of erythropoietin, thus maintaining DO2 at the expense of increasing viscosity of blood.
What is the most important immediate adaptation?
The most important immediate adaptation is hyperventilation and thus increase in minute ventilation due to the decrease in PaO2, this via stimulation of the peripheral chemoreceptors (central chemoreceptors are not sensitive to falls in PaO2). This results in a respiratory alkalosis.
Why is PVR elevated?
PVR becomes increased chronically due to hypoxic vasoconstriction. The CO returns to normal with a few days of acclimatization. With continued presence at altitude, vital capacity and FRC is unchanged from baseline, but MV remains elevated.
