
How does pressure affect melting and boiling point? When we apply pressure on a substance, its melting point increases or decreases depending upon the change of volume which occurs during the phase change of that substance. The boiling point of liquids always increases when pressure is applied on that liquid.
What is the effect of decrease pressure on melting point?
You will observe that the wire cuts through the ice, but the ice does not break into two pieces. The pressure of the wire lowers the melting point of the ice below it, and thus the ice melts. The wirer therefore cuts through. As the pressure is released, the melting point is again raised and the water formed resolidifies.
Does the melting point increase as pressure is applied?
When we apply pressure on a substance, its melting point increases or decreases depending upon the change of volume which occurs during the phase change of that substance. The boiling point of liquids always increases when pressure is applied on that liquid.
What factors affect the melting point?
What factors affect the melting point of an ionic compound?
- Inter Molecular Forces. When the attraction between molecules are weaker, we can say that the inter molecular forces are weak.
- Shape of Molecules. Shapes of molecules also affect the melting of a substance.
- Size of Molecules.
- Other Factors.
How pressure effects the melting points of solids?
When pressure is applied on the surface of a normal solid, expansion is suppressed and melting is delay. Thus, the melting point of a normal solid is raised. Abnormal solids, like ice and bismuth, contract on melting into liquids. When pressure is applied on the surface of such a solid, the change into the liquid is assisted.
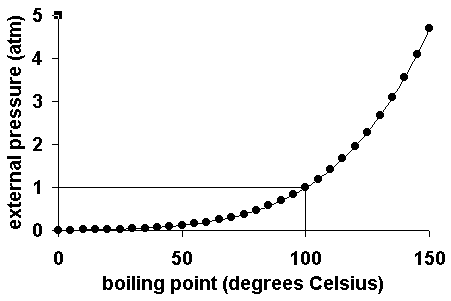
How does pressure affect melting point?
Pressure and its Effect on Melting Point In most cases, increased pressure increases the melting point for a material. This means that under high-pressure conditions, you would need to apply more heat to cause a material to melt.
How does pressure affect boiling point?
Boiling. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid will boil.
How does low pressure affect melting point?
Melting point: The temperature at which a solid turns into a liquid. The melting point of water is dependent of the pressure above the ice (solid water), and the melting point or freezing temperature decreases with increasing pressure. By definition 0 °C is at the melting point of water at 1 atmosphere pressure.
Does increased pressure raise boiling point?
Atmospheric Pressure and Boiling The pressure of gas above a liquid affects the boiling point. In an open system this is called atmospheric pressure. The greater the pressure, the more energy required for liquids to boil, and the higher the boiling point.
Why does water melt under pressure?
The H-O bond relaxation determines the melting point and the O:H freezing and boiling. Figure 4 shows that both air pressure and water boiling point drops as one goes higher. The lowered pressure lengthens and softens the O:H nonbond and the H-O bond relaxes reversely.
How can change in pressure affect the boiling point of a compound?
As pressure increases, the energy required to boil a liquid increases because boiling point is the point where the vapour pressure equals or exceeds the atmospheric pressure.
Why does water boil faster at lower pressure?
At higher pressures (such as the pressure generated in a pressure cooker), the temperature must be higher before the vapor pressure reaches the surrounding pressure, so water under pressure boils at a higher temperature.
How does pressure affect temperature?
For example, if air pressure increases, the temperature must increase. If air pressure decreases, the temperature decreases.
What are the factors affecting boiling point?
Boiling point is the temperature where the liquid turns into vapour and vapour pressure is equal to the atmospheric pressure.The boiling point of a liquid depends on pressure, vapour pressure and molecular weight.
Why does melting point increase as pressure increases?
This is because, due to the increased pressure, it is easier for molecules to remain in solid form (this is what the whole liquefying a gas works on).
Why is boiling a higher temperature?
Boiling: Pressure allows water to stay squished in liquid form. Adding temperature to the liquid makes the molecules bounce more violently, until many break away into gas form. So higher temperatures mean boiling. Less pressure allows the liquids to break away into gas form easier. So lower pressure means lower boiling temperatures.
Why does water melt?
Water is one of a few special substances for which the pressure lowers the temperature of transition . The basic reason is that water actually expands when it goes from the liquid to solid phase. …it is perhaps most readily explained using LeChatelier's principle.
What happens to the volume of a metal in equilibrium?
For metals in equilibrium condition, their volume expands during melting.
What happens when you apply pressure to a material?
Generally, if pressure is applied on a material, the structure or arrangement of lattice changes. The material ( any shape) becomes compact during the application of pressure. Now, if we try to melt that material, it will take more energy to melt, as meltng increases the surface area and makes ions or lattice free.
When we increase the surrounding pressure, does the vapour pressure increase?
So, when we increase the surrounding pressure, vapour pressure increases, and hence there is a lesser gap between the vapour pressure and the atmospheric pressure (note that atmospheric pressure remains constant). So, vapour pressure can be equal to the surrounding pressure at a lesser temperature.
What happens to a lattice when pressure is applied?
Generally, if pressure is applied on a material, the structure or arrangement of lattice changes. The material ( any shape) becomes compact during the application of pressure.
Why does the boiling point of a liquid increase when pressure is applied?
This is because the molecules of the liquid will require comparatively more energy to turn into gaseous state when pressure is applied on that substance. Share.
What happens to the melting point of a solid if the volume of the liquid phase is greater than the volume of?
To be specific, if the volume of the substance's liquid phase is greater than the volume of the solid phase, its melting point will increase upon the increase of volume. Again, if the volume of the substance's liquid phase is less than the volume of the solid phase, its melting point will decrease upon the increase of volume.
