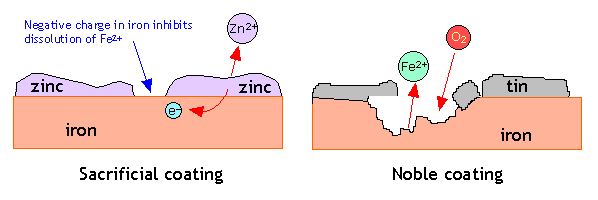
Sacrificial protection: In this method, surface of iron is covered with layer of more active metal like zinc. This active metal loses electrons (undergoes oxidation) in preference to iron and hence, prevents the rusting of iron. Zinc metal is generally used for protecting iron and the process is called galvanization.
Which metal is used to prevent iron from rusting?
A more reactive metal than Iron, e.g. Zinc, is used as the Zinc metal would oxidize in the place of iron, thus preventing Iron from rusting. How can sacrificial protection prevent iron rusting?
What is meant by sacrificial protection?
- Answers Sacrificial protection is basically attaching a piece of metal that is more reactive that iron to the object made of iron. This more reactive metal, commonly magnesium or zinc, will corrode in the place of iron.
Why doesn't gold get oxidized in sacrificial protection?
Sacrificial protection is only effective if the metal used for sacrificial protection is more reactive than Iron in the reactivity series. Since gold is one of the least reactive metals in the reactivity series, it would not get oxidized in the place of Iron. A more reactive metal than Iron, e. g. Zinc, is used as the Zinc.
Which metals are used as sacrificial metals?
A reactivity series lists metals in order of how reactive they are. Magnesium and zinc are often used as sacrificial metals. They are more reactive than iron and lose their electrons in preference to iron.

How does sacrificial prevent iron from rusting?
Sacrificial protection Iron can be protected from rusting if it is in contact with a more reactive metal, such as zinc. The more reactive metal oxidises more readily than iron, so it 'sacrifices' itself while the iron does not rust. Once the sacrificial metal has corroded away, it can simply be replaced.
How does the sacrificial protection of iron work?
The sacrificial process occurs when the more reactive metal, which may be attached using copper wires, donates its electrons to the iron and replaces those lost when iron has oxidized, thereby reverting the iron back to its original state.
How does sacrificial coating prevent corrosion?
Sacrificial coatings contain certain elements, such as zinc or aluminum, which corrode sacrificially to ensure that the component's substrate remains corrosion free. Because the coating corrodes instead of the component to which they are applied, the structural integrity of the substrate is maintained.
What is sacrificial protection used for?
Zinc corrodes in preference to steel and sacrifices itself to protect the steel, hence hot dip galvanizing will provide this sacrificial protection. The corrosion products from the zinc are deposited on the steel resealing it from the atmosphere and therefore stopping corrosion.
What is sacrificial metal for iron?
zincCorrosion is occurring at a great rate but its not iron but rather zinc that is rusting. Simply put, the zinc is sacrificing itself to protect the iron hence the name sacrificial anode.
What metal can be used in the sacrificial protection of iron?
Magnesium and zinc are often used as sacrificial metals. They are more reactive than iron and lose their electrons in preference to iron. This prevents iron from losing its electrons and becoming oxidised.
What is the difference between Galvanising and sacrificial protection?
Galvanising is coating the iron with a layer of zinc in order to prevent it from rusting. However, sacrificial protection is attaching a piece of zinc to the iron object.
Why is zinc coating on iron called sacrificial anode?
The zinc serves as a sacrificial anode so that even if the coating is scratched, the exposed steel will still be protected by the remaining zinc. The zinc protects its base metal by corroding before iron.
What is the difference between sacrificial and non sacrificial coatings?
1. What is the difference between a sacrificial and a non sacrificial anti graffiti coating? A sacrificial coating withstands one or two graffiti removals, whereas according to the Australian Standard a non sacrificial has to be able to withstand 10 plus removals of graffiti. 2.
Which is the best method to prevent rusting?
9 Ways to Prevent RustUse an Alloy. Many outdoor structures, like this bridge, are made from COR-TEN steel to reduce the effects of rust. ... Apply Oil. ... Apply a Dry Coating. ... Paint the Metal. ... Store Properly. ... Galvanize. ... Blueing. ... Powder Coating.More items...•
Which of the following is used to protect iron from rusting?
Galvanization prevents rusting of iron.
What are the two methods to prevent rusting?
Solution : The rusting of iron can be prevented by painting, oiling, greasing or varnishing its surface.
(2) Galvanisation is another method of protecting iron from rusting by coating iron with a thin layer of zinc.
(3) Corrosion of iron is prevented by coating iron with non-xcorrosive substance like carbon.
How was Harry Potter protected from Voldemort?
Lily Potter sacrificed her life in order to protect her infant son, Harry from Lord Voldemort. This placed Harry under magical protection, so that when Voldemort cast the Killing Curse at Harry in turn, the spell backfired, leaving him unharmed (save for a lightning-shaped scar on his forehead) and Voldemort bodiless.
How will you prevent iron or other metals from rusting?
9 Ways to Prevent RustUse an Alloy. Many outdoor structures, like this bridge, are made from COR-TEN steel to reduce the effects of rust. ... Apply Oil. ... Apply a Dry Coating. ... Paint the Metal. ... Store Properly. ... Galvanize. ... Blueing. ... Powder Coating.More items...•
How does Galvanising prevent rusting?
Galvanizing protects from rust in a number of ways: It forms a barrier that prevents corrosive substances from reaching the underlying steel or iron. The zinc serves as a sacrificial anode so that even if the coating is scratched, the exposed steel will still be protected by the remaining zinc.
What is the difference between Galvanising and sacrificial protection?
Galvanising is coating the iron with a layer of zinc in order to prevent it from rusting. However, sacrificial protection is attaching a piece of zinc to the iron object.