
As the concentration of substrate increased there were more substrate molecules to bond with the active site of the catalase enzyme. Because there was this increase in the substrate concentration each time, there was a greater likelihood that the substrate would bind with the active site and carry out the reaction.
How does substrate concentration affect enzyme reaction rates?
Substrate concentration: Increasing substrate concentration also increases the rate of reaction to a certain point. Once all of the enzymes have bound, any substrate increase will have no effect on the rate of reaction, as the available enzymes will be saturated and working at their maximum rate.
How does hydrogen peroxide affect catalase?
Problem Statement:
- Objectives: To determine the presence of catalase on the rate of reaction of hydrogen peroxide.
- Aim:
- Hypothesis: The higher the concentration of enzyme, the higher the rate of reaction until a maximum velocity is reached.
- Techniques:
- Materials:
- Apparatus:
- Variables: By using different mass of blended potato at 1g, 2g, 3g and 4g. ...
What is the effect of temperature on catalase?
Reference
- (Jones, 2009).
- "Introduction to Enzymes. " Factors Affecting Enzyme Activity (). N. p. , n. d. Web. 16 Nov. 2012.
- "Effect of Temperature on Enzyme Activity. " Effect of Temperature on Enzyme Activity. N. p. , n. d. Web. 16 Nov. 2012.
What is the optimum pH for catalase?
In the case of catalase, the optimum pH is approximately pH 7.0. That is, catalase works best at a neutral pH. If the solution is too acidic (low pH value) or too basic (high pH value) the catalase is inactive and no longer functions as an enzyme.
How does substrate concentration affect catalase enzyme activity?
As the concentration of substrate increased there were more substrate molecules to bond with the active site of the catalase enzyme. Because there was this increase in the substrate concentration each time, there was a greater likelihood that the substrate would bind with the active site and carry out the reaction.
How does substrate concentration affect enzyme activity a level?
Substrate Enzyme Concentration In the presence of a given amount of enzyme, the rate of enzymatic reaction increases as the substrate concentration increases until a limiting rate is reached, after which further increase in the substrate concentration produces no significant change in the reaction rate.
How does substrate concentration affect enzyme activity example?
If an enzyme is to be used to determine the concentration of substrate in a sample (e.g. glucose oxidase is used to measure plasma glucose), then the substrate must be the limiting factor, and the concentration of substrate must be below Km, so that the rate of formation of product increases steeply with increasing ...
How does enzyme substrate concentration affect enzymes?
0:223:51How does Substrate Concentration affect Enzyme Activity | BiologyYouTubeStart of suggested clipEnd of suggested clipSo the concentration of substrate affects the enzyme activity. The more substrate concentration theMoreSo the concentration of substrate affects the enzyme activity. The more substrate concentration the faster the enzymes will work this is shown in this graph the substrate concentration the rate of the
What happens when substrate concentration increases?
Increasing Substrate Concentration increases the rate of reaction. This is because more substrate molecules will be colliding with enzyme molecules, so more product will be formed.
What factors affect catalase activity?
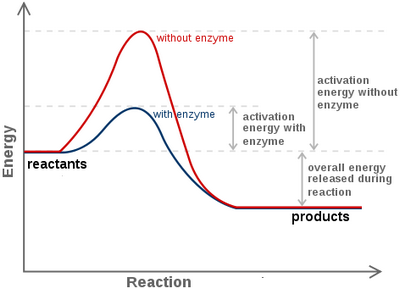
The rate at which an enzyme works is influenced by several factors including the concentration of substrate (hydrogen peroxide in the case of catalase), temperature, pH, salt concentration and the presence of inhibitors or activators.
Why is substrate concentration important?
Since substrate concentration is an important factor in deciding enzyme activity, it is used in calculating the rate of an enzymatic reaction and binding affinity (how much the enzyme likes the substrate). The rate of an enzyme reaction is the amount of substrate converted to product over a period of time.
What happens when substrate concentration decreases?
This means that as the enzyme concentration decreases, the reaction rate will decrease. In most biological environments, the concentration of the enzyme is lower than the concentration of the substrate. The relationship between enzyme concentration and enzyme activity is directly proportional.
What does a high substrate concentrate mean?
Increasing substrate concentration increases the frequency with which the enzyme and substrate collide. As a result enzyme-substrate complexes form more quickly and the rate of reaction increases.
How does concentration affect enzyme activity?
Enzyme concentration The activity of an enzyme increases as the concentration of the enzyme increases. This is because more enzymes are available to bind to the substrate. In turn, the reaction speed increases. As long as there is a substrate to bind to, increasing enzyme concentration will speed up the reaction.
What would happen if the catalase was used up?
If the catalase was used up then another potato would have to be crushed and this could produce catalase of a totally different concentration which would lead to inaccuracies in the experiment making this an unfair test.
What is catalase used for?
Enzymes such as Catalase are protein molecules which are found in living cells. They are used to speed up specific reactions in the cells. They are all very specific as each enzyme just performs one particular reaction. Catalase is an enzyme found in food such as potato and liver. It is used for removing Hydrogen Peroxide from the cells.
What happens when you double the concentration of hydrogen peroxide?
When the concentration of Hydrogen Peroxide is increased, the rate of reaction increases at a directly proportional rate until the concentration of Hydrogen Peroxide reaches about 16%. If you double the concentration of Hydrogen Peroxide then the rate of reaction doubles as well.
Why is hydrogen peroxide able to speed up the decomposition of hydrogen peroxide?
It is able to speed up the decomposition of Hydrogen Peroxide because the shape of it's active site matches the shape of the Hydrogen Peroxide molecule. This type of reaction where a molecule is broken down into smaller pieces is called an anabolic reaction.
How to make different concentrations of hydrogen peroxide?
The different concentrations of Hydrogen Peroxide are made by adding tap water to the 20% Hydrogen Peroxide in the correct amounts. The table below shows what amounts of Hydrogen Peroxide and water are needed to make the solutions. Concentration Of Hydrogen Peroxide. Volume Of Hydrogen Peroxide (cm3)
How to measure the volume of hydrogen peroxide?
1. Add 2cm3 of yeast to one test tube. Add 4cm3 of hydrogen peroxide solution at a concentration of 20% to the other test tube. Use a pipette to measure out the volumes. It is very important to accurately measure the amounts of Hydrogen Peroxide, Yeast and water to ensure a fair test. 2.
What happens when the amount of substrate molecules added exceeds the number of active sites available?
Once the amount of substrate molecules added exceeds the number of active sites available then the rate of reaction will no longer go up. This is because the maximum number of reactions are being done at once so any extra substrate molecules have to wait until some of the active sites become available.
What happens when substrate concentration increases?
As the enzyme molecules become saturated with substrate, this increase in reaction rate levels off. The rate of an enzyme-catalyzed reaction increases with an increase in the concentration of an enzyme.
Does substrate concentration affect the rate of reaction?
Substrate concentration: Increasing substrate concentration also increases the rate of reaction to a certain point. Once all of the enzymes have bound, any substrate increase will have no effect on the rate of reaction, as the available enzymes will be saturated and working at their maximum rate. Click to see full answer.
Is catalase dependent on substrate concentration?
In fact, the catalase reaction is dependent on the substrate concentration. However, at some point you will reach a substrate concentration at which the enzyme gets saturated and becomes the limiting factor. In this case you have to add more enzyme to speed up the reaction again.
Introduction
Hypothesis
- I believe that as the concentration of the hydrogen peroxide (substrate) decreases, the rate of reaction will decrease as well. This is because as there are progressively fewer molecules of hydrogen peroxide there will be fewer collisions between the substrate and enzyme molecules (catalase in yeast), leading to a decrease in enzyme-substrate compl...
Preliminary Work
- As a result of my preliminary work, I have identified problems that may occur in my main investigation, such as timing, measuring and keeping variables that I am not investigating constant. Here are the proposed solutions to the problems I identified. Control Temperature With a Water Bath In the main procedure, I will control the temperature with a water bath in order to cr…
Independent Variable
- The independent variable (the factor that I manipulate) will be the concentration of the hydrogen peroxide. I intend to use a pipette in order to make the concentrations of 100%, 90%, 80%, 70%, 60% and 50%. I will do this by making each mixture up to 100cm3, so for example, the 90% concentrated solution will consist of 90cm3 of hydrogen peroxide and 10cm3 water. I will put th…
Dependent Variable
- The dependent variable (the one I intend to measure) is the volume of gas produced in each reaction. This will vary as a direct result of the different concentrations of hydrogen peroxide.
Controlled Variables
- The controlled variables are the other factors which must be kept constant. One such variable will be the mass of yeastfor each experiment (0.2g). I will make sure that I measure 0.2g of yeast as accurately as I can using the balance. The balance has a mechanism whereby it can be made level (perfectly balanced) regardless of the angle of the desk or counter it is placed on. I have ex…
Apparatus
- Conical flask
- 20 vols hydrogen peroxide
- Water
- Yeast
Method
- Measure out the concentrations of hydrogen peroxide (100%, 90%, 80%, 70%, 60% and 50%) by adding different volumes of water to make up 100cm3. For example, the 80% concentrated solution will consis...
- Place the six conical flasks in a water bath at 25oC to create a constant external temperature and dissipate heat energy. Do this first to ensure that the mixtures have enough time to reac…
- Measure out the concentrations of hydrogen peroxide (100%, 90%, 80%, 70%, 60% and 50%) by adding different volumes of water to make up 100cm3. For example, the 80% concentrated solution will consis...
- Place the six conical flasks in a water bath at 25oC to create a constant external temperature and dissipate heat energy. Do this first to ensure that the mixtures have enough time to reach a const...
- Grind the yeast into a powder using a pestle and mortar. Note:Grind up more than required, so you can use the same (ground) yeast for each experiment. This will also be fairer than grinding the yea...
- Set up your apparatus.
Safety
- Hydrogen peroxide, if inhaled or in contact with the skin or eyes, can be very dangerous and toxic. For this reason, I will take the following safety precautions: 1. Wear safety goggles and gloves whenever handling the hydrogen peroxide. 2. Keep hair tied back at all times. 3. Do not wear any jewellery or articles of clothing that may come into contact with the hydrogen peroxide. 4. Clean …
Graphs
- Predict what the graph will show. I believe that the graph will start off steep in all the reactions, but steepest in the 100% concentration of hydrogen peroxide and gradually decreasing as the concentration of hydrogen peroxide decreases. This is because there will be more collisions between the enzyme and substrate molecules resulting in more enzyme-substrate complexes. T…