By limiting the orbiting electrons to a series of circular orbits having discrete radii, Bohr could account for the series of discrete wavelengths in the emission spectrum of hydrogen. Light, he proposed, radiated from hydrogen atoms only when an electron made a transition from an outer orbit to one closer to the nucleus.
What is the Bohr model of the hydrogen atom?
Merits of Bohr’s Theory
- Bohr’s model explains the stability of the atom. ...
- Bohr’s theory successfully explains the atomic spectrum of hydrogen.
- The theory explains the hydrogen spectrum and the spectra of one electron species such as H e +, L i 2 + and B e 3 +, etc.
What is the Bohr radius of hydrogen?
What is Bohr’s radius of hydrogen atom? It is named after Niels Bohr, due to its role in the Bohr model of an atom. Its value is 5.29177210903 (80)×10−11 m….Bohr radius. Does hydrogen have a high atomic radius?
What is Bohr atomic theory?
The Bohr atomic model theory considers electrons to have both a known radius and orbit i.e. known position and momentum at the same time, which is impossible according to Heisenberg. The Bohr atomic model theory made correct predictions for smaller sized atoms like hydrogen, but poor spectral predictions are obtained when larger atoms are considered.
What is the atomic model of hydrogen?
Bohr’s atomic model hydrogen emission spectra. Bohr explained that electrons can be moved into different orbits with the addition of energy. When the energy is removed, the electrons return back to their ground state, emitting a corresponding amount of energy – a quantum of light, or photon.
Q.1. How do you draw a Bohr model of hydrogen?
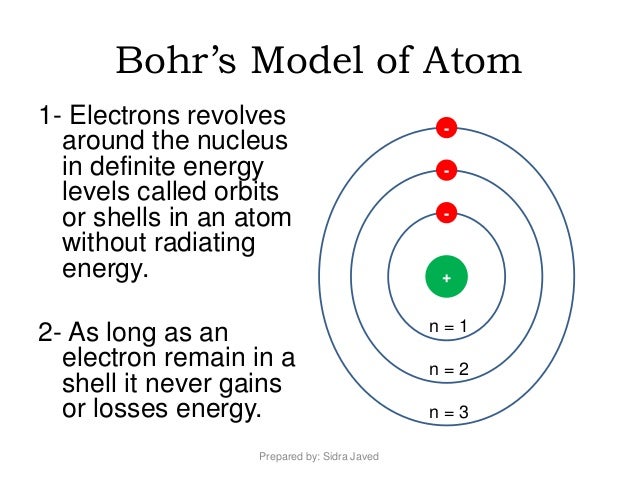
Ans: Bohr’s model of hydrogen is drawn as below.
Q.2. What are the three principles of Bohr’s model?
Ans: The electrons occupy certain orbits around the nucleus. As long as the electron revolves in a particular orbit, the electron neither gains nor...
Q.3. How do you find the Bohr model of an atom?
Ans: Electrons revolve around the nucleus in specified circular paths called orbits or shells. These orbits are numbered as 1,2,3,4…, etc., represe...
Q.4. What are the limitations of the Bohr hydrogen model?
Ans: Bohr’s theory fails to explain the spectra of multielectron atoms. It does not explain the splitting of spectral lines into a group of finer l...
Q.5. What is the formula for Bohr’s model?
Ans: The formula for Bohr’s model is l=nh2π l or mvr= Angular momentum n= principal quantum number h= Planck’s constant.
Q.6. What four assumptions did Bohr make about the electronic structure of the atom?
Ans: The four assumptions Bohr made are listed below: Bohr’s model explains the stability of the atom. The electron revolves in a stationary orbit,...
Q.7. How does the Bohr model of the hydrogen atom explain the spectrum of hydrogen[EI1]?
Ans: Bohr tells us that the electrons in the Hydrogen atom can only occupy discrete orbits around the nucleus (not at any distance from it but cert...
What is Bohr’s Model for Hydrogen Atom?
He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. In the model, electrons orbit the nucleus in atomic shells.
Which theory explains the atomic spectrum of hydrogen?
Bohr’s theory successfully explains the atomic spectrum of hydrogen. The theory explains the hydrogen spectrum and the spectra of one electron species such as He +, Li2 + and Be3 +, etc. The experimentally determined frequencies of spectral lines are in close agreement with those calculated by Bohr’s theory .
What is the Bohr model?
Bohr model for Hydrogen-Like Atoms. The Bohr model of the atom was replaced by the Quantum Mechanics model based upon the Schrodinger equation in the 1920s. The great success of the Bohr model had been in explaining the spectra of hydrogen-like (single electron around a positive nucleus) atoms.
What are the postulates of Bohr's model of atom?
The main postulates of Bohr’s model of atom are as follows: The electrons move around the nucleus with definite velocity in a certain fixed closed circular path called orbits (or) shells. These shells are numbered as one, two, three, four or termed as K, L, M, N from the nucleus.
Which atom model was based on the idea that the nucleus revolves around the electron?
However, the concept of the nucleus as enunciated by the Rutherford Atomic Model was retained. Bohr postulated that Planck’s Quantum theory applies to the electron that revolves around the nucleus. On this basis, Bohr proposed this theory to explain the structure of an atom.
Which scientist proposed the Hydrogen atom model?
However, it was not wholly correct. It needed slight modifications that were made by a legendary scientist named Neil’s Bohr. Bohr recognised that the lines in an atomic spectrum are related to the arrangement of electrons in that atom.
What is the new model of an atom called?
This new model is called Bohr’s model of an atom. Bohr’s model is based on applying the quantum theory of radiation for revolving electrons around ...
Which Bohr model isn't applicable for systems more than one electron?
Bohr’s Model of the Hydrogen Atom isn’t applicable for systems more than one electron.
What are the limitations of Bohr's model?
Limitations of the Bohr Model of the Hydrogen Atom: 1 Bohr’s model doesn’t work well for complex atoms. 2 It couldn’t explain why some spectral lines are more intense than others. 3 It could not explain why some spectral lines split into multiple lines in the presence of a magnetic field. 4 The Heisenberg’s uncertainty principle contradicts Bohr’s idea of electrons existing in specific orbits with a known radius and velocity.
How is the energy of an electron in the shell explained?
According to Bohr’s calculation, the energy for an electron in the shell is given by the expression: The hydrogen spectrum is explained in terms of electrons absorbing and emitting photons to change energy levels , where the photon energy is: Bohr’s Model of the Hydrogen Atom isn’t applicable for systems more than one electron.
What was Bohr's assumption?
The assumption was the quantization of the structure of atoms. Bohr’s proposed that electrons orbited the nucleus in specific orbits or shells with a fixed radius. Only those shells with a radius provided by the equation below were allowed, and it was impossible for electrons to exist between these shells.
How do electrons absorb energy?
According to Bohr’s model, an electron would absorb energy in the form of photons to get excited to a higher energy level. After escaping to the higher energy level, also known as the excited state, the excited electron is less stable, and therefore, would rapidly emit a photon to come back to a lower, more stable energy level. The energy of the emitted photon is equal to the difference in energy between the two energy levels for a specific transition. The energy can be calculated using the equation
Which principle contradicts Bohr's idea of electrons existing in specific orbits with a known radius and?
The Heisenberg’s uncertainty principle contradicts Bohr’s idea of electrons existing in specific orbits with a known radius and velocity. Although the modern quantum mechanical model and the Bohr Model of the Hydrogen Atom may seem vastly different, the fundamental idea is the same in both.
Which model of atomic theory asserted that electrons revolved around a positively charged nucleus?
But Bohr supported the planetary model, which asserted that electrons revolved around a positively charged nucleus just like the planets around the sun.
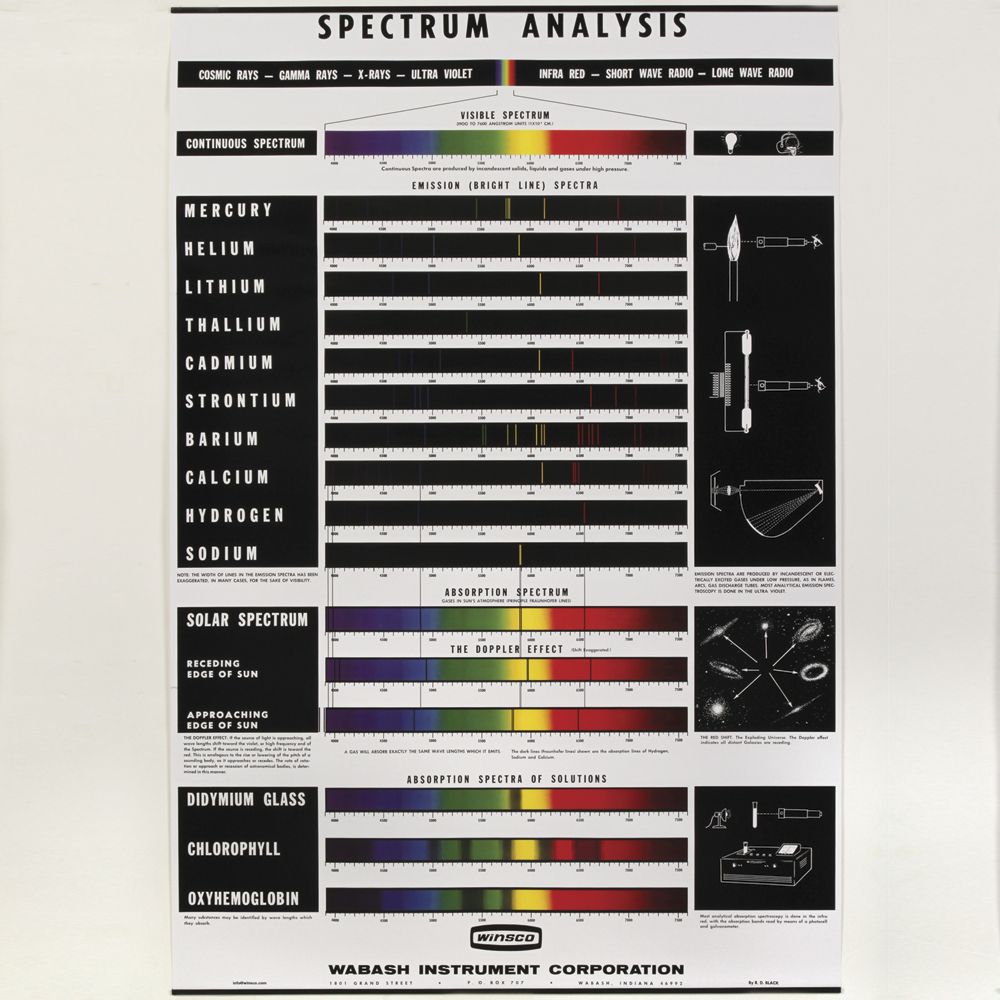
How does Bohr explain the hydrogen line emission spectrum?
How Bohr explanation of the hydrogen line emission spectrum led to the quantum mechanical model of the atom. A spectrum is the ‘picture’ you get when light interacts with atoms or molecules. A spectrum is usually a plot of how much light is absorbed or emitted versus the wavelength or frequency of light. For example, when white light ...
Why did Bohr's atomic model not explain the many lines present in the spectra of elements with?
The first was that Bohr’s atomic model could not explain the many lines present in the spectra of elements with more than one electron. These elements include all the elements after hydrogen on the periodic table. Another was that Bohr thought as if there were actually well-defined orbits in an atom. Because of these problems, a new model was needed to explain the line emission spectra of all elements.
What did Schrodinger do with the De Broglie hypothesis?
In 1926, another scientist called Schrodinger did just that. He applied the De Broglie hypothesis to develop a wave equation to describe how an electron behaved within a hydrogen atom. From the wave equation, Schrodinger generated wave functions. These wave functions, which are solutions to the wave equations, are used to predict the energy states of an electron, and the probability of finding it somewhere within an atom.
How did Bohr relate the energy of the electron to the radius of the orbit?
To account for this, Bohr related the energy of the electron to the radius of the orbit by bringing up Planck’s idea that energy is emitted in packets (quantized). Bohr believed that electrons could only circulate around the nucleus in orbits of certain radii.
How does hydrogen absorb energy?
If we apply this understanding to our energized tube of hydrogen gas, we can say that as the atoms of hydrogen molecules absorb energy, their electrons become excited (excited state), allowing the electrons jump from a lower energy level to a higher energy level. As they return to their original state (ground state), they emit the extra energy they initially absorbed as light. When this light is passed through a glass prism onto a white surface, we see a few colored lines that fall within the visible region of the electromagnetic spectrum similar to the one below.
What is the Rydberg constant?
where the constant 2.179 x 10 -18 J, is called the Rydberg constant and n, represents the principal quantum number or energy level. The principal quantum number starts from n = 1 to infinity. Here is an illustration of Bohr’s atomic model;
What is line emission spectrum?
Because of this, a line emission spectrum is like the “fingerprint” of the element, which we can use to identify the element.
What is the Bohr model of hydrogen?
Bohr Model of the Hydrogen Atom: To describe the structure of atoms, the first model was proposed by J.J Thomson. According to this, the positively charged particle is distributed throughout the atom, and negatively charged particles are embedded in it like seeds of watermelon. Soon after it failed, Rutherford proposed an atomic model; with the help of the alpha-scattering experiment, he proved that all positively charged particles are present at the centre of the nucleus. He also proposed that the electron revolves around the nucleus in a varying radius. He was not able to explain the stability of atoms and discrete wavelengths in the hydrogen spectrum. Then Bohr gives a model for hydrogen-like atoms and explains the stability and discrete wavelengths in the hydrogen spectrum.
How did Niels Bohr modify the Rutherford model?
Niels Bohr made some modifications to the Rutherford model by adding the ideas of the newly developing quantum hypothesis. To explain the position, motion of the electron, and stability of the atom, he gave three postulates.
Does the spectral line of hydrogen atoms talk about the fine structure?
It does not talk about the fine structure of the spectral lines of hydrogen atoms.