How does the buffer system work in the human blood? Buffering system of blood When any acidic substance enters the bloodstream, the bicarbonate In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula HCO⁻₃. In chemistry, hydronium is the common name for the aqueous cation H3O+, the type of oxonium ion produced by protonation of water. It is the positive ion present when an Arrhenius acid is dissolved in water, as Arrhenius acid molecules in solution give up a proton to the surrounding water molecules.Bicarbonate
Hydronium
What are the buffer systems of blood?
The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The kidneys help control acid-base balance by excreting hydrogen ions and generating bicarbonate that helps maintain blood plasma pH within a normal range.
What is the function of a buffer in blood?
Proteins and other compounds in blood act as buffers, which help to regulate the pH of body tissues. Blood also helps to regulate the water content of body cells. If you have had blood test, it was likely drawn from a superficial vein in your arm, which was then sent to a lab for analysis.
What are the acid-base buffer systems in the blood?
The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The kidneys help control acid-base balance by excreting hydrogen ions and generating bicarbonate that helps maintain blood plasma pH within a normal range.
How do buffers in the blood affect the pH?
pH range: Buffering agents keep blood pH between 7.38 and 7.42. Acid–base imbalances in the blood’s pH can be altered by changes in breathing to expel more CO 2 and raise pH back to normal. Hydrogen ions (H+) are carried in the blood along with oxygen and carbon dioxide.
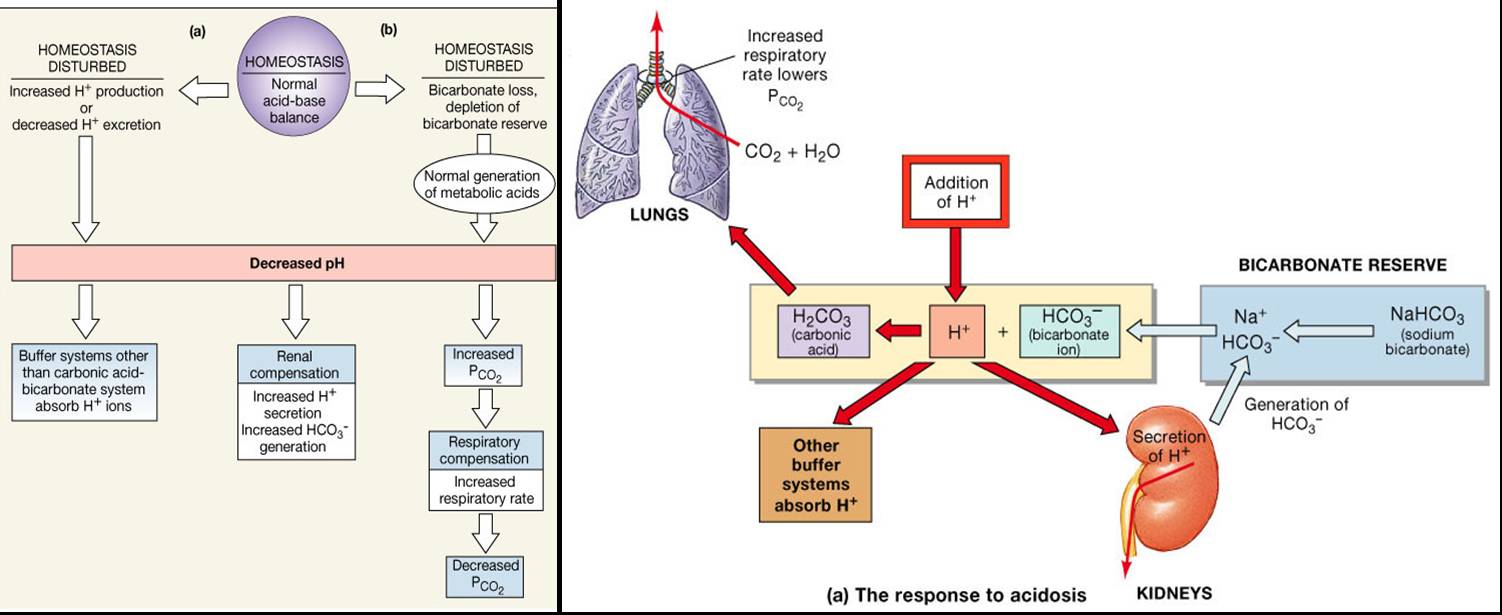
What regulates the pH of human blood?
Buffer regulates the pH of human blood. How does the buffer work in our body and what is chemical composition of buffer?
When is H+ added to blood?
In short When H+ is added to the blood as a result of metabolic processes, the amount of HCO3- (relative to the amount of CO2) decreases; however, the amount of the change is tiny compared to the amount of HCO3- present in the blood. This optimal buffering occurs when the pH is within approximately 1 pH unit from the pK value for the buffering system, i.e., when the pH is between 5.1 and 7.1.
Can hemoglobin bind to Fe?
Hemoglobin protein can reversibly bind either H + (to the protein) or O 2 (to the Fe of the heme group). For a more complex and obviously more complete description see for example: https://www.khanacademy.org/test-prep/mcat/chemical-processes/acid-base-equilibria/a/chemistry-of-buffers-and-buffers-in-blood.
What is the buffering system of blood?
Buffering system of blood. Maintaining a constant blood pH is critical for the proper functioning of our body. The buffer that maintains the pH of human blood involves a carbonic acid (H CO) - bicarbonate ion (HCO) system. Diagram carbonic acid - bicarbonate ion system in human blood.
How does a buffer work?
Buffer, as we have defined, is a mixture of a conjugate acid-base pair that can resist changes in pH when small volumes of strong acids or bases are added.
How do we prepare a buffer?
mixing a large volume of a weak acid with its conjugate base (eg. acetic acid – acetate ion, CH COOH – CH COO)
What happens when carbonic acid enters the bloodstream?
On the other hand, when a basic substance enters the bloodstream, carbonic acid reacts with the hydroxide ions producing bicarbonate ions and water. Bicarbonate ions are already a component of the buffer. In this manner, the hydroxide ions are removed from blood, preventing the pH of blood from becoming basic.
What happens when a strong acid is added to a buffer?
When a strong acid is added, the acetate ions neutralize the hydronium ions producing acetic acid (which is already a component of the buffer). On the other hand, when a strong base is added, acetic acid consumes the hydroxide ions producing acetate ions (which is also already a constituent of the buffer).
What is phosphate buffer?
Phosphate buffer is a very commonly used buffer in research labs. It falls under the second category. It's made up of a weak base (HPO) and its conjugate acid (H PO ). The pH of a phosphate buffer is usually maintained at a physiological pH of 7.4.
What is the best buffering ratio?
The best buffering will occur when the ratio of [HA] to [A] is almost 1:1. In that case pH = pK. Buffers are considered to be effective when the ratio of [HA] to [A] ranges anywhere between 10:1 and 1:10.
Uses of Buffer: Maintaining pH of Blood
A buffer is an acid-base mixture that is able to resist changes in pH when either a strong acid or a strong base are added to it. A buffer consists of either a weak acid and its conjugate base or a weak base and its conjugate acid. Carbonic acid is the weak acid that acts as a buffer in blood, and its conjugate base is the bicarbonate ion.
Bicarbonate Buffer System: How it Works in Blood
How do carbonic acid and bicarbonate ions act as a blood buffer system? The bicarbonate buffer system works in the blood to maintain the needed pH, or concentration of H + ions.
Bicarbonate Buffer System Equation
The bicarbonate buffer system equation is the equation of the two-way reactions that take place in the bloodstream as the pH starts to increase or decrease. Remember how the bicarbonate buffer system works:
