
How long does it take for methylnaltrexone to work?
One 2016 study found that 45.9 percent of people taking methylnaltrexone experienced a rescue-free bowel movement within 4 hours of the first dose. By the fifth week of taking the drug, around 70 percent of participants experienced three or more rescue-free bowel movements per week.
How is methylnaltrexone excreted from the body?
Methylnaltrexone is excreted primarily as the unchanged drug in the urine and feces. Active renal secretion of methylnaltrexone is suggested by renal clearance of methylnaltrexone that is approximately 4-to 5-fold higher than creatinine clearance.
What is the half life of methylnaltrexone in Relistor?
Following oral administration of a single 450 mg dose of Relistor tablets, concentrations of methylnaltrexone declined in multiphasic manner with a terminal half-life (t1/2) of approximately 15 hours.
How quickly does Relistor (methylnaltrexone) work for constipation?
Relistor (methylnaltrexone) doesn't work quickly for everyone, however, so you may have to take laxatives if you don't have success after 4 hours. The oral form of Relistor (methylnaltrexone) doesn't work as reliably or quickly, but taking it every day will help you have more bowel movements per week.
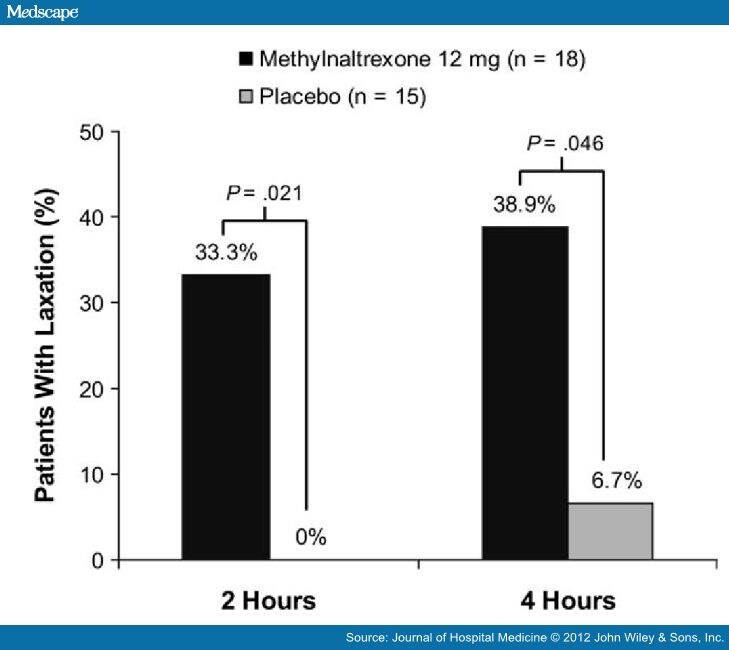
How quickly does Relistor (methylnaltrexone) work?
After getting a Relistor (methylnaltrexone) injection, you can expect to have a bowel movement within 4 hours. Some people may even be able to have...
Is Relistor (methylnaltrexone) a laxative?
Relistor (methylnaltrexone) helps with laxation, which is the medical term for having a bowel movement. Unlike traditional laxatives, which are typ...
Is Relistor (methylnaltrexone) a narcotic?
Relistor (methylnaltrexone) isn't a narcotic or controlled substance, since it doesn't have potential for abuse. Relistor (methylnaltrexone) is use...
How often can you take Relistor (methylnaltrexone)?
If you are using Relistor (methylnaltrexone) to treat opioid-related constipation and you don't have cancer, you should take this medication once d...
How should I take Relistor (methylnaltrexone)?
If you're taking the oral form of Relistor (methylnaltrexone), you'll want to take it with water on an empty stomach at least 30 minutes before you...
What is the purpose of methylnaltrexone?
Methylnaltrexone is used to treat constipation caused by opioid (narcotic) pain medications in people with chronic (ongoing) pain that is not caused by cancer but may be related to a previous cancer or cancer treatment. Methylnaltrexone is in a class of medications called peripherally acting mu-opioid receptor antagonists.
What is the name of the drug that is used in Contrave?
Be sure to mention any of the following: alvimopan (Entereg), naldemedine (Symproic), naloxegol (Movantik), naloxone (Evzio, Narcan, in Bunavail, Suboxone, Zubsolv), or naltrexone (Vivitrol, in Contrave, Embeda). Your doctor may need to change the doses of your medications or monitor you carefully for side effects.
Can you stop taking methylnaltrexone?
If you stop taking opioid medications, you should stop taking methylnaltrexone as well. You should stop taking other laxative medications when you start taking methylnaltrexone. However, be sure to let your doctor know if methylnaltrexone does not work for you after taking it for 3 days.
Can methylnaltrexone cause runny nose?
If you experience any of these symptoms, stop taking methylnaltrexone and call your doctor immediately: severe diarrhea. severe abdominal pain. Methylnaltrexone may cause other side effects. Call your doctor if you have any unusual problems while taking this medication.
What is methylnaltrexone bromide?
Methylnaltrexone ( MNTX, brand name Relistor ), used in form of methylnaltrexone bromide ( INN, USAN, BAN ), is a medication that acts as a peripherally acting μ-opioid receptor antagonist that acts to reverse some of the side effects of opioid drugs such as constipation without significantly affecting pain relief or precipitating withdrawals. Because MNTX is a quaternary ammonium cation, it cannot cross the blood–brain barrier, and so has antagonist effects throughout the body, counteracting effects such as itching and constipation, but without affecting opioid effects in the brain such as pain relief. However, since a significant fraction (up to 60%) of opioid analgesia can be mediated by opioid receptors on peripheral sensory neurons, particularly in inflammatory conditions such as arthritis, traumatic or surgical pain, MNTX may increase pain under such circumstances.
What is the name of the drug that Wyeth developed?
In December 2005, Wyeth and Progenics entered into an exclusive, worldwide agreement for the joint development and commercialization of methylnaltrexone for the treatment of opioid-induced side effects, including constipation and post-operative ileus (POI), a prolonged dysfunction of the gastrointestinal tract following surgery.
Why did Leon Goldberg decline morphine?
Struggling with the pain of prostatic cancer that had metastasized to his bones, the man was now declining the morphine he required for analgesia because of constipation.
Does methylnaltrexone cause withdrawal symptoms?
Furthermore, as methylnaltrexone cannot cross the blood–brain barrier, it does not reverse the pain-killing properties of opioid agonists or cause withdrawal symptoms, but since a small portion of analgesia comes from the peripheral opioid receptors, it can increase pain from inflammatory conditions such as arthritis.
Does methylnaltrexone affect the brain?
Methylnaltrexone is unable to enter the brain primarily because it carries a positive charge on its nitrogen atom. This is the primary characteristic that makes methylnaltrexone behave differently than naltrexone.
Does MNTX cause pain?
However, since a significant fraction (up to 60%) of opioid analgesia can be mediated by opioid receptors on peripheral sensory neurons, particularly in inflammatory conditions such as arthritis, traumatic or surgical pain, MNTX may increase pain under such circumstances.
Is methylnaltrexone in human milk?
There is no information regarding the presence of methylnaltrexone in human milk, the effects on the breastfed infant, or the effects on milk production. Methylnaltrexone is present in rat milk [see Data ]. Because of the potential for serious adverse reactions, including opioid withdrawal, in breastfed infants, advise women that breastfeeding is not recommended during treatment with RELISTOR.
Is methylnaltrexone bromide overdosed?
During clinical trials of RELISTOR administered orally and subcutaneously, one accidental case of methylnaltrexone bromide overdose was reported and no adverse events were reported as a result of the overdosage.
Does methylnaltrexone inhibit CYP?
In vitro, methylnaltrexone did not significantly inhibit or induce the activity of cytochrome P450 (CYP) isozymes CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2C19, or CYP3A4. In addition, methylnaltrexone did not induce CYP2E1.
Is methylnaltrexone a selective antagonist?
Methylnaltrexone is a selective antagonist of opioid binding at the mu-opioid receptor. As a quaternary amine, the ability of methylnaltrexone to cross the blood-brain barrier is restricted. This allows methylnaltrexone to function as a peripherally-acting mu-opioid receptor antagonist in tissues such as the gastrointestinal tract, thereby decreasing the constipating effects of opioids without impacting opioid-mediated analgesic effects on the central nervous system.
Does Relistor cause pain?
The most common side effects of RELISTOR tablets in people with long-lasting (chronic) pain that is not caused by cancer include: stomach-area (abdomen) pain, diarrhea, headache, swelling or a feeling of fullness or pressure in your abdomen, sweating, anxiety, muscle spasms, runny nose, and chills.
How long does it take for a bowel movement to go away after taking methylnaltrexone?
Methylnaltrexone is a highly effective medicine. found that 45.9 percent of people taking methylnaltrexone experienced a rescue-free bowel movement within 4 hours of the first dose. By the fifth week of taking the drug, around 70 percent of participants experienced three or more rescue-free bowel movements per week.
How does relistor work?
How Relistor works. Relistor helps reduce opioid-induced constipation. Opioids, such as morphine, stop pain by numbing pain receptors in the brain. However, they also numb the receptors in the digestive tract.
Why was Relistor developed?
Scientists developed Relistor to treat opioid-induced constipation without taking away from the pain-relieving effect of opioid medication. In 1978, colleagues at the University of Chicago started looking for ways to help people who would not take morphine for pain due to unbearable constipation. They wanted to find a medication ...
What is the effect of relistor on the gut?
Relistor, or methylnaltrexone bromide, blocks the numbing effects of opioids in the intestines. The drug binds to the receptors in the gut, blocking morphine’s effects on them, and so they are no longer numb. This means that the muscles in the gut can process feces normally once more.
When was Relistor approved?
The Food and Drug Administration (FDA) approved Relistor for the treatment of opioid-induced constipation in 2008. Last medically reviewed on February 25, 2019. Constipation. Cancer / Oncology. Pain / Anesthetics.
What is the name of the drug that Boehringer Ingelheim made?
A compound called N-methyl-naltrexone (MNTX), that the German pharmaceutical company Boehringer Ingelheim made, was promising. In 2005, following tests, two pharmaceutical companies signed an agreement to develop the drug and sell methylnaltrexone.
Does relistor enter the brain?
Relistor does not enter the brain, so morphine continues to enact a painkilling effect. If the drug was to enter the brain, it would cancel out morphine’s painkilling effects, leaving the individual in pain again.
What is the steady state volume of distribution of methylnaltrexone?
The steady-state volume of distribution (Vss) of methylnaltrexone is approximately 1.1 L/kg. The fraction of methylnaltrexone bound to human plasma proteins is 11% to 15%, as determined by equilibrium dialysis.
How much methylnaltrexone bromide should I give to a CD1 mouse?
Two-year oral carcinogenicity studies have been conducted with methylnaltrexone bromide in CD-1 mice at doses up to 200 mg/kg/day (about 81 times the subcutaneous maximum recommended human dose (MRHD) of 12 mg/day based on body surface area) in males and 400 mg/kg/day (about 162 times the subcutaneous MRHD of 12 mg/day) in females and in Sprague Dawley rats at oral doses up to 300 mg/kg/day (about 243 times the subcutaneous MRHD of 12 mg/day). The 200 mg/kg/day and 400 mg/kg/day doses in male and female mice are about 2.2 and 4.4 times, respectively, the oral MRHD of 450 mg/day, and the 300 mg/kg/day dose in rats is about 6.5 times the oral MRHD of 450 mg/day, based on body surface area. Oral administration of methylnaltrexone bromide for 104 weeks did not produce tumors in mice and rats.
How to use a vial?
Use one hand to hold the vial steady. Use your other hand to insert the needle straight down into the rubber top of the vial ( See Figure K). Do not insert it at an angle. This may cause the needle to bend or break. You will feel some resistance as the needle passes through the rubber top.
Is methylnaltrexone bromide negative?
Methylnaltrexone bromide was negative in the Ames test, chromosome aberration tests in Chinese hamster ovary cells and human lymphocytes, in the mouse lymphoma cell forward mutation tests and in the in vivo mouse micronucleus test.
Is methylnaltrexone in breast milk?
There is no information regarding the presence of methylnaltrexone in human milk, the effects on the breastfed infant, or the effects on milk production. Methylnaltrexone is present in rat milk [see Data]. Because of the potential for serious adverse reactions, including opioid withdrawal, in breastfed infants, advise women that breastfeeding is not recommended during treatment with Relistor.
Is methylnaltrexone a selective antagonist?
Methylnaltrexone is a selective antagonist of opioid binding at the mu-opioid receptor. As a quaternary amine, the ability of methylnaltrexone to cross the blood-brain barrier is restricted. This allows methylnaltrexone to function as a peripherally-acting mu‑opioid receptor antagonist in tissues such as the gastrointestinal tract, thereby decreasing the constipating effects of opioids without impacting opioid-mediated analgesic effects on the central nervous system.

Overview
Methylnaltrexone (MNTX, brand name Relistor), used in form of methylnaltrexone bromide (INN, USAN, BAN), is a medication that acts as a peripherally acting μ-opioid receptor antagonist that acts to reverse some of the side effects of opioid drugs such as constipation without significantly affecting pain relief or precipitating withdrawals. Because MNTX is a quaternary ammoni…
Medical uses
Methylnaltrexone is approved for the treatment of opioid-induced constipation in chronic non cancer pain or when ordinary laxatives have failed.
Mechanism of action
Methylnaltrexone is a peripheral acting mu-opioid receptor antagonist, and does not cross the blood brain barrier. Methylnaltrexone has restricted access through the blood brain barrier because it is a quaternary amine, which carries a positive charge when in a solution and more has polarity with lower lipid solubility than a lot of the opioid agonists used for pain treatment. The peripheral action of methylnaltrexone makes it effective for decreasing the constipating effects …
Side effects
The most common side effects for methylnaltrexone include:
• Abdominal pain
• Dizziness
• Vomiting
• Nausea
History
In 1978, a dying friend and colleague presented the late University of Chicago pharmacologist Leon Goldberg with a clinical challenge. Struggling with the pain of prostatic cancer that had metastasized to his bones, the man was now declining the morphine he required for analgesia because of constipation. Research on opioids which would target only the sub-types of receptors associated with pain relief and not with side effects had seen little success outside of in-vitro m…
Society and culture
On April 1, 2008, Progenics and Wyeth announced that Health Canada has approved methylnaltrexone for the treatment of opioid-induced constipation. It was later approved by the US FDA on April 24, 2008.
As of 2010, methylnaltrexone is supplied as an injection in trays containing seven one-dose vials containing 0.6 mL of solution. Each tray also contains seven 12 mm (0.47 in) 1 mL 27 gauge nee…
See also
• Loperamide - an μ-opioid receptor agonist that doesn't cross the BBB in significant amounts, and treats diarrhea (in contrast to methynaltrexone, a Mu-opioid receptor antagonist that doesn't cross the BBB, avoiding opiate withdrawal effects in patients, while treating constipation)
• Naloxegol (trade names Movantik and Moventig) - another peripherally selective opioid antagonist used to treat opioid-induced constipation
Further reading
• Holzer P (February 2007). "Treatment of opioid-induced gut dysfunction". Expert Opinion on Investigational Drugs. 16 (2): 181–94. doi:10.1517/13543784.16.2.181. PMID 17243938. S2CID 9838569.
• Yuan CS, Foss JF (September 2000). "Oral methylnaltrexone for opioid-induced constipation". JAMA. 284 (11): 1383–4. doi:10.1001/jama.284.11.1383. PMID 10989399.