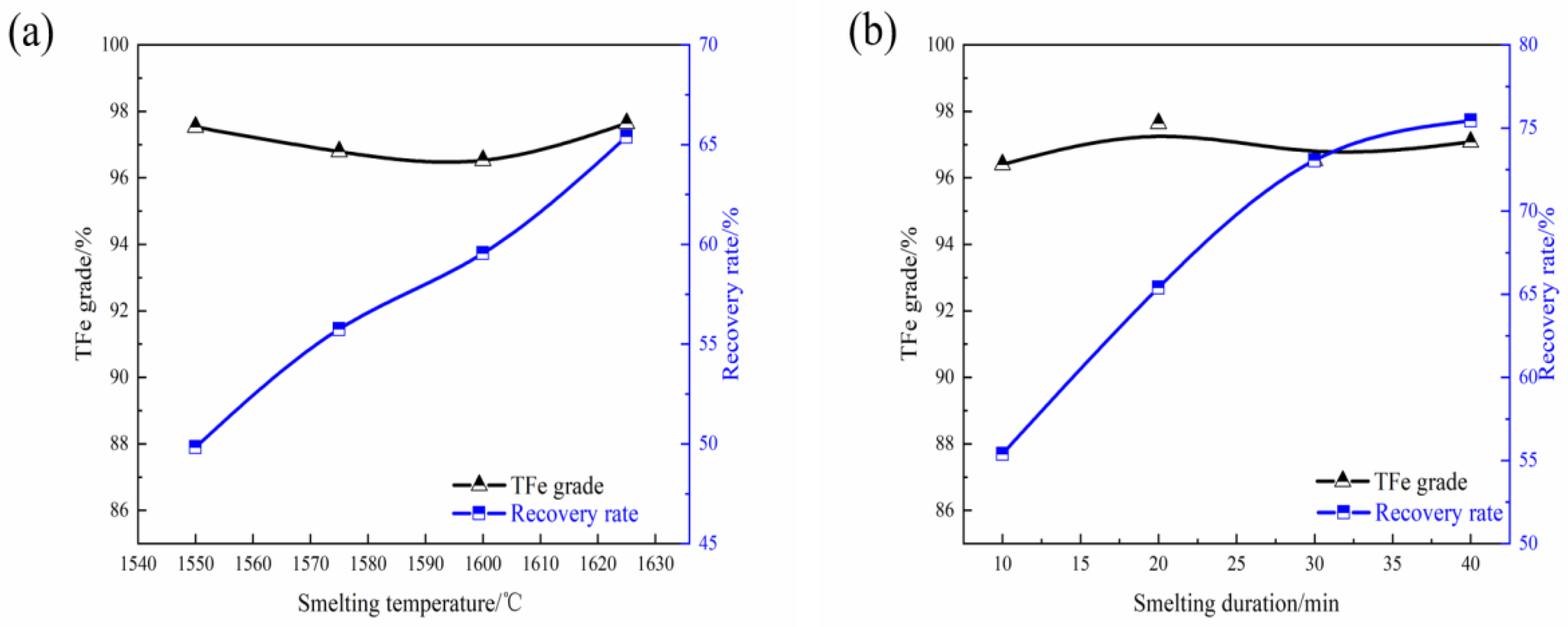
Is aluminum dangerous to human?
Dangers of Aluminum. (1) It is a poison that acts on the nervous system and has been linked to several serious health problems. Aluminum accumulates in the kidneys, brain, lungs, liver and thyroid where it competes with calcium for absorption and can affect skeletal mineralization.
Is aluminum toxic in body?
Exposure to high levels of aluminum may lead to potentially harmful toxicity. Old age and reduced kidney function are two most common risk factors associated with aluminum poisoning. According to an article published in Medscape.com, aluminum toxicity is commonly found in patients with renal impairment.
What are the effects of aluminum?
Aluminum has long been established in medical applications as, e.g., an adjuvant in vaccines and an agent against pathological hyperhidrosis with a low side-effect profile (1, 2).In recent years, however, there has been more focus on the at times highly uncritical public debate about the neurotoxic effect of aluminum and its potential carcinogenic effect.
What is the process of making aluminum?
- Material Preheating – The billet gets cut to the correct length. ...
- Extrusion – Once the billet heats up, it gets coated with a lubricant. ...
- Cooling – Once the material goes through the dies, it gets extruded and cooled using air or water, sometimes both.
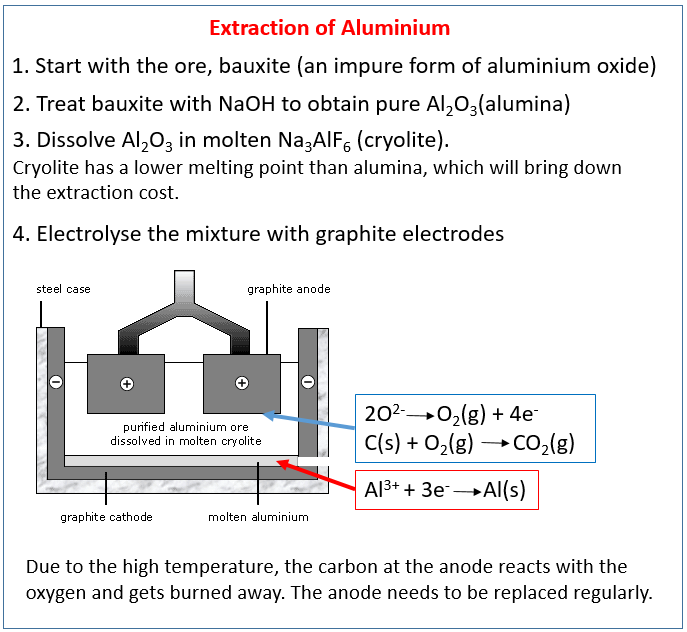
Why is aluminium extracted by electrolysis?
Electrolysis is used to extract aluminium from its ore as aluminium is more reactive than carbon therefore cannot be extracted by carbon. Any metal in the reactivity series above carbon must be extracted using electrolysis as it to too reactive.
How is aluminium extracted from its ore bauxite by Hall's process?
The Hall–Héroult process is the major industrial process for smelting aluminium. It involves dissolving aluminium oxide (alumina) (obtained most often from bauxite, aluminium's chief ore, through the Bayer process) in molten cryolite, and electrolyzing the molten salt bath, typically in a purpose-built cell.
How is aluminium extracted from its ore Class 10?
Extraction of aluminium Aluminium ore is called bauxite . The bauxite is purified to produce aluminium oxide, a white powder from which aluminium can be extracted. The extraction is done by electrolysis. The ions in the aluminium oxide must be free to move so that electricity can pass through it.
Which process is used for aluminium extraction?
The Hall-Heroult process is widely used in the extraction of aluminium.
How is aluminium obtained from bauxite?
Bauxite ore (Al2O3, impure) is first purified to form pure Al2O3. And then it goes through the Electrolysis process to give Aluminium metal at the cathode. Aluminium cannot be extracted by heating alone because aluminium is very reactive and it hangs on with oxygen and does not form aluminium metal.
What are the steps involved in the extraction of aluminium from bauxite?
Extraction of Aluminium from Bauxite It is done by hand–picking, grinding, etc. Bauxite ore generally contains ferric oxide and silica as impurities. Dressing of Bauxite ore is done by crushing and pulverising. To remove ferric oxide impurities from the Bauxite, a magnetic separation method is used.
How aluminum is refined by Hall's method electrolytic method?
In the Hall-Héroult smelting process, a nearly pure aluminum oxide compound called alumina is dissolved at 950 °C (1,750 °F) in a molten electrolyte composed of aluminum, sodium, and fluorine; this is electrolyzed to give aluminum metal at the cathode and oxygen gas at the anode.
How is aluminium produced from bauxite?
Pure aluminium oxide, called alumina, is extracted from bauxite via a process called refining, composed of two steps: a digestion process, using caustic soda, which allows the separation of aluminium hydroxide from the so-called “bauxite residue”, followed by a calcination step which removes the water content in the ...
How is Aluminium extracted from its ore?
Bauxite is an important ore of aluminium. Bauxite is crushed, leached and then subjected to electrolysis to get aluminium.
Why is Aluminium extracted from alumina?
Aluminium is more reactive than carbon and has more affinity towards oxygen. So aluminium can not be reduced by using carbon. Electrolysis is the b...
What are the properties of Aluminium?
Pure aluminium has high electrical conductivity and is flexible, ductile, corrosion-resistant. Aluminium is being used in an ever-increasing number...
What are the types of Aluminium?
There are two main classifications, casting alloys and wrought alloys, both further subdivided into the heat-treatable and non-heat-treatable categ...
Why is Aluminium ore purified?
Ore is a chemical substance in which metal is in the combined form along with gangue. Bauxite is an ore of aluminium which is hydrated aluminium ox...
Can Aluminium be extracted by smelting?
Smelting is a process by which metal is obtained from its ore by using heat and a reducing agent. Being electropositive, aluminium has a high affin...
1. Where are the largest deposits of Bauxite found?
Bauxite maybe sort of earthy in nature but its large or abundant deposits are found in northern South America, India, Caribbean islands, Malaysia,...
2. What are the common alloying elements in Aluminium?
The Aluminium that is used widely in recent times is mixed with some other elements to increase its strength. However, the most common alloying ele...
3. How is Aluminium produced from Bauxite?
Aluminium is produced from Bauxite in a two-step process that consists of refining to get alumina and smelting to produce Aluminium from that obtai...
4. What is the most common Aluminium Ore?
The most common Aluminium Ore is Bauxite, which is a sedimentary rock, and it is a rock that contains around 52 per cent of Aluminium oxide with ce...
5. Are the largest producers of Bauxite the top producers of Aluminium?
Even though Aluminium is gathered from Bauxite, the largest producers of Bauxite are certainly not the topmost producer of the metal, Aluminium. Th...
What is the electrolysis method of alumina?
Electrolysis by Hall – Heroult Method – Alumina is highly stable oxide and melts at 2050℃ that’s why alumina cannot be directly electrolyzed. Its electrolysis is done with cryolite (3 parts by weight) and fluorspar (1 part by weight). In this process for electrolysis an iron tank lined with heat resistant material and has a sloping floor, provided with an outlet for tapping molten aluminium metal is used. Gas carbon or graphite are used as cathode and thick carbon rods are used as anode. Coke powder covering is used to prevent burning of carbon anodes and to prevent heat loss from molten electrolyte. A direct current of 100 A is passed through the electrolyte and the temperature is maintained at 950℃. In this process sodium, calcium and aluminium ions are formed which migrates towards the cathode. However, only aluminium ions reach to the cathode due to their lower position in the electrochemical series. Thus, pure aluminium get deposited at cathode and melts due to 950℃ temperature of the electrolyte, as it is heavier than electrolyte, so it gets deposited at the base of the electrolytic tank. While at anode nascent oxygen is formed which reacts with carbon of coke and forms carbon mono oxide which reacts with atmospheric oxygen and forms carbon dioxide. Although nascent oxygen formed at anode reacts with carbon of carbon-anode as well. That’s why carbon – anodes are consumed gradually and need to be replaced time to time.
What is the process of extracting aluminium from bauxite?
Extraction of Aluminium from Bauxite. The science of extracting pure metals by economically effective methods from their ores is called metallurgy. Metallurgy of aluminium or extraction process of aluminium from its ore involves various methods. Mostly aluminium is extracted from its ore called bauxite.
What is the most common form of aluminium?
Generally, Aluminium ores are found in the form of its oxides. Bauxite is the most common aluminium ore and Hall – Heroult process is the major industrial process for extraction of aluminium from its oxide alumina. In this article we will discuss occurrence and extraction of aluminium in detail.
How is aluminium extracted?
Mostly aluminium is extracted from its ore called bauxite. The extraction of aluminium from its ore involves following steps –. Dressing or Concentration of the bauxite ore by Hall’s method. Electrolysis by Hall – Heroult Method. Dressing or concentration of the bauxite ore– Bauxite is generally found as an impure form of aluminium oxide.
What are the resources of the Earth?
We get minerals and ores in abundance in the earth’s crust. Some ores have been proven themselves a great resource for mankind. Such as iron obtained from ore of iron (Hematite) built the foundation of industrial revolution. On the other hand, aluminium was a crucial strategic resource for aviation during World War I and World War II. Still, aluminium metal dominates in the various fields of the market due to its unique properties and easy and cost - effective extraction. Generally, Aluminium ores are found in the form of its oxides. Bauxite is the most common aluminium ore and Hall – Heroult process is the major industrial process for extraction of aluminium from its oxide alumina. In this article we will discuss occurrence and extraction of aluminium in detail.
How is impure bauxite converted to sodium aluminate?
Step 1. Conversion of impure bauxite into sodium aluminate – The ore is fused to red heat with sodium carbonate and formation of sodium aluminate takes place. Reaction involved is given below –
How is dressing of bauxite ore done?
Bauxite ore generally contains ferric oxide and silica as impurities. Dressing of bauxite ore is done by crushing and pulverizing.
How is aluminium oxide extracted?
The extraction is done by electrolysis, but first the aluminium oxide must be melted so that electricity can pass through it. However, aluminium oxide has a very high melting point (over 2,000°C) so it would be expensive to melt it.
What is the negative electrode of an electrolysis cell made of?
The diagram shows an aluminium oxide electrolysis cell. Both the negative electrode (cathode) and positive electrode (anode) are made of graphite, which is a form of carbon.
What is the process of electrolysis?
Electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given electrolyte. Aluminium is one metal which is extracted from its ore by this method.
Why is aluminum so expensive?
Aluminium is the most abundant metal on Earth, but it is expensive, largely because of the amount of electricity used in the extraction process.
Why should electrolysis be near the coast?
near the coast to allow for the import of raw materials. near roads and railway lines to allow for the product to be taken to where it is needed.
What happens to the oxygen in a positive electrode?
This oxygen reacts with the carbon of the positive electrodes, forming carbon dioxide, so they gradually burn away. As a result, the positive electrodes have to be replaced frequently. This adds to the cost of the process.
Where does molten aluminium sink?
The molten aluminium sinks to the bottom of the cell, where it is tapped off.
Largest Producer For Aluminium
China Hongqiao is currently the largest aluminium smelter and producer, with a combined annual capacity of up to 3.61 million tonnes. In 2010 production of aluminium was 16 million metric tonnes to 2019, which is now 36 million tonnes.
How Is Aluminium Produced?
As previously mentioned, Bauxite is a raw material and is usually found in clay-like soil. Throughout the years, Aluminium production has been most common in Queensland, Australia or throughout Northern Territory. Australia is the worlds largest producer of Bauxite with 20% OF GLOBAL Alumina and 30% of global Bauxite.
How is alumina released from bauxite?
In the first step of the process, the alumina is released from the bauxite when it is crushed and dissolved in sodium hydroxide. The resulting solution travels through a number of filters and separators to remove insoluble particles (leaving a liquefied sodium aluminate). In the second step, the alumina is recovered from this solution ...
What is the process of removing alumina from bauxite?
The bauxite is blasted loose and washed to remove any excess clay. The second step is known as the Bayer process , which involves a combination of a chemical extraction and a mechanical separation to extract alumina (the base from of aluminium) from the bauxite.
How to extract aluminium from ore?
The process to extract the aluminium from its ore is a seemingly complex and time consuming one, and it goes as follows: Bauxite Mining. The first step in the process is to mine the bauxite from the earth, mostly from underground deposits (the largest ones can be found in Southern Europe and Hungary). The bauxite is blasted loose and washed ...
Where is aluminium made?
As you can see, the process used to extract aluminium from the bauxite ore it originates in is fairly complex and requires a number of different machines in order to be completed. Whilst the biggest aluminium manufacturing plants are located in Europe, they do exist here in Australia, just not on the same large scale due to our smaller deposits.
